Biotransformation: Impact and Application of Metabolism in Drug Discovery
- PMID: 33214818
- PMCID: PMC7667663
- DOI: 10.1021/acsmedchemlett.0c00202
Biotransformation: Impact and Application of Metabolism in Drug Discovery
Abstract
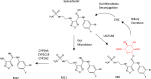
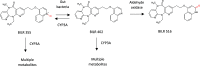
Biotransformation has a huge impact on the efficacy and safety of drugs. Ultimately the effects of metabolism can be the lynchpin in the discovery and development cycle of a new drug. This article discusses the impact and application of biotransformation of drugs by mammalian systems, microorganisms, and recombinant enzymes, covering active and reactive metabolites, the impact of the gut microbiome on metabolism, and how insights gained from biotransformation studies can influence drug design from the combined perspectives of a CRO specializing in a range of biotransformation techniques and pharma biotransformation scientists. We include a commentary on how biology-driven approaches can complement medicinal chemistry strategies in drug optimization and the in vitro and surrogate systems available to explore and exploit biotransformation.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
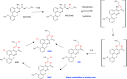
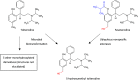
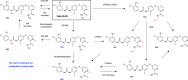
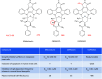
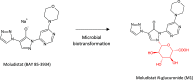
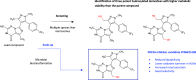

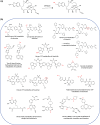
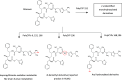


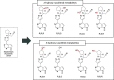
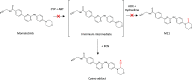
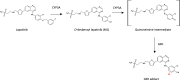

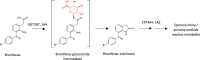
References
-
- Safety Testing of Drug Metabolites Guidance for Industry. U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER). March 2020. Pharmacology/Toxicology, Revision 2. https://www.fda.gov/media/72279/download (accessed 2020-08-12).
-
- Gu C.; Artelsmair M.; Elmore C. S.; Lewis R. J.; Davis P.; Hall J. E.; Dembofsky B. T.; Christoph G.; Smith M. A.; Chapdelaine M.; Sunzel M. Late-occurring and long-circulating metabolites of GABAAα2,3 receptor modulator AZD7325 involving metabolic cyclization and aromatization: relevance to MIST analysis and application for patient compliance. Drug Metab. Dispos. 2018, 46 (3), 303–315. 10.1124/dmd.117.078873. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

