Dual-Target Inhibitors of the Folate Pathway Inhibit Intrinsically Trimethoprim-Resistant DfrB Dihydrofolate Reductases
- PMID: 33214838
- PMCID: PMC7667824
- DOI: 10.1021/acsmedchemlett.0c00393
Dual-Target Inhibitors of the Folate Pathway Inhibit Intrinsically Trimethoprim-Resistant DfrB Dihydrofolate Reductases
Abstract
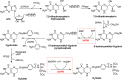
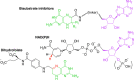
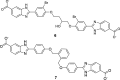
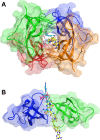
Trimethoprim (TMP) is widely used to treat infections in humans and in livestock, accelerating the incidence of TMP resistance. The emergent and largely untracked type II dihydrofolate reductases (DfrBs) are intrinsically TMP-resistant plasmid-borne Dfrs that are structurally and evolutionarily unrelated to chromosomal Dfrs. We report kinetic characterization of the known DfrB family members. Their kinetic constants are conserved and all are poorly inhibited by TMP, consistent with TMP resistance. We investigate their inhibition with known and novel bisubstrate inhibitors of 6-hydroxymethyl-7,8-dihydropterin pyrophosphokinase (HPPK). Importantly, all are inhibited by the HPPK inhibitors, making these molecules dual-target inhibitors of two folate pathway enzymes that are strictly microbial.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Discovery of Highly Trimethoprim-Resistant DfrB Dihydrofolate Reductases in Diverse Environmental Settings Suggests an Evolutionary Advantage Unrelated to Antibiotic Resistance.Antibiotics (Basel). 2022 Dec 7;11(12):1768. doi: 10.3390/antibiotics11121768. Antibiotics (Basel). 2022. PMID: 36551425 Free PMC article.
-
Structure-Based Design of Dimeric Bisbenzimidazole Inhibitors to an Emergent Trimethoprim-Resistant Type II Dihydrofolate Reductase Guides the Design of Monomeric Analogues.ACS Omega. 2019 Jun 10;4(6):10056-10069. doi: 10.1021/acsomega.9b00640. eCollection 2019 Jun 30. ACS Omega. 2019. PMID: 31460098 Free PMC article.
-
The Bacterial Genomic Context of Highly Trimethoprim-Resistant DfrB Dihydrofolate Reductases Highlights an Emerging Threat to Public Health.Antibiotics (Basel). 2021 Apr 13;10(4):433. doi: 10.3390/antibiotics10040433. Antibiotics (Basel). 2021. PMID: 33924456 Free PMC article.
-
Trimethoprim and brodimoprim resistance of gram-positive and gram-negative bacteria.J Chemother. 1993 Dec;5(6):458-64. J Chemother. 1993. PMID: 8195838 Review.
-
Trimethoprim alone for treatment of urinary tract infection.Rev Infect Dis. 1982 Mar-Apr;4(2):366-71. doi: 10.1093/clinids/4.2.366. Rev Infect Dis. 1982. PMID: 7051236 Review.
Cited by
-
A dual-target herbicidal inhibitor of lysine biosynthesis.Elife. 2022 Jun 20;11:e78235. doi: 10.7554/eLife.78235. Elife. 2022. PMID: 35723913 Free PMC article.
-
Discovery of Highly Trimethoprim-Resistant DfrB Dihydrofolate Reductases in Diverse Environmental Settings Suggests an Evolutionary Advantage Unrelated to Antibiotic Resistance.Antibiotics (Basel). 2022 Dec 7;11(12):1768. doi: 10.3390/antibiotics11121768. Antibiotics (Basel). 2022. PMID: 36551425 Free PMC article.
-
Crystal structure of the plasmid-encoded R67 dihydrofolate reductase complexed with Congo red an amyloid binding dye.Sci Rep. 2025 Feb 12;15(1):5212. doi: 10.1038/s41598-025-89539-3. Sci Rep. 2025. PMID: 39939735 Free PMC article.
-
A conserved SH3-like fold in diverse putative proteins tetramerizes into an oxidoreductase providing an antimicrobial resistance phenotype.Philos Trans R Soc Lond B Biol Sci. 2023 Feb 27;378(1871):20220040. doi: 10.1098/rstb.2022.0040. Epub 2023 Jan 11. Philos Trans R Soc Lond B Biol Sci. 2023. PMID: 36633286 Free PMC article.
-
relA Inactivation Converts Sulfonamides Into Bactericidal Compounds.Front Microbiol. 2021 Sep 27;12:698468. doi: 10.3389/fmicb.2021.698468. eCollection 2021. Front Microbiol. 2021. PMID: 34646242 Free PMC article.
References
-
- Shaw G. X.; Li Y.; Shi G.; Wu Y.; Cherry S.; Needle D.; Zhang D.; Tropea J. E.; Waugh D. S.; Yan H.; Ji X. Structural enzymology and inhibition of the bi-functional folate pathway enzyme HPPK-DHPS from the biowarfare agent Francisella tularensis. FEBS J. 2014, 281 (18), 4123–4137. 10.1111/febs.12896. - DOI - PMC - PubMed
-
- Giguère S.; Prescott J. F.; Dowling P. M.. Antimicrobial therapy in veterinary medicine; John Wiley & Sons Inc, 2013; Vol. 47, pp 256–257.
LinkOut - more resources
Full Text Sources

