Single Agent and Synergistic Activity of Maritoclax with ABT-263 in Nasopharyngeal Carcinoma (NPC) Cell Lines
- PMID: 33214852
- PMCID: PMC7652248
- DOI: 10.21315/tlsr2020.31.3.1
Single Agent and Synergistic Activity of Maritoclax with ABT-263 in Nasopharyngeal Carcinoma (NPC) Cell Lines
Abstract
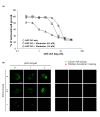
The BCL-2 anti-apoptotic proteins are over-expressed in many cancers and hence are attractive therapeutic targets. In this study, we tested the sensitivity of two Nasopharyngeal Carcinoma (NPC) cell lines HK1 and C666-1 to Maritoclax, which is reported to repress anti-apoptotic protein MCL-1 and BH3 mimetic ABT-263, which selectively inhibits anti-apoptotic proteins BCL-2, BCL-XL and BCL-w. We investigated the sensitisation of the NPC cell lines to these drugs using the SYBR Green I assay and 3D NPC spheroids. We report that Maritoclax repressed anti-apoptotic proteins MCL-1, BCL-2, and BCL-XL in a dose- and time-dependent manner and displayed a single agent activity in inhibiting cell proliferation of the NPC cell lines. Moreover, combination of Maritoclax and ABT-263 exhibited synergistic antiproliferative effect in the HK1 cells. Similar results were obtained in the 3D spheroids generated from the HK1 cells. More notably, 3D HK1 spheroids either treated with single agent Maritoclax or combination with ABT-263, over 10 days, did not develop resistance to the treatment rapidly. Collectively, the findings illustrate that Maritoclax as a single agent or combination with BH3 mimetics could be potentially useful as treatment strategies for the management of NPC.
Protein anti-apoptotik BCL-2 diekspres pada tahap yang tinggi dalam kebanyakan kanser dan merupakan sasaran terapeutik kanser yang menarik. Dalam kajian ini, sensitiviti dua titisan sel kanser nasofarinks diuji, iaitu HK1 dan C666-1 kepada Maritoclax yang dilaporkan menurunkan tahap protein anti-apoptotik MCL-1 dan BH3 mimetik ABT-263 yang merencat protein anti-apoptotik BCL-2, BCL-XL dan BCL-w. Sensitiviti sel kanser nasofarinks kepada dadah ini disiasat menggunakan ujian SYBR Green I dan sferoid 3D kanser nasofarink. Di sini kami melaporkan bahawa Maritoclax mengurangkan tahap MCL-1, BCL-2 dan BCL-XL mengikut konsentrasi drug dan tempoh rawatan dan Maritoclax memaparkan aktiviti agen tunggal dalam menghalang percambahan sel kanser nasofarinks. Selain itu, gabungan Maritoclax dan ABT-263 menghalang percambahan sel kanser nasofarinks HK1 secara sinergistik. Keputusan yang sama diperolehi dengan sferoid 3D sel HK1. Lebih penting, sferoid 3D sel HK1, sama ada dirawat dengan agen tunggal Maritoclax atau gabungan dengan ABT-263 selama 10 hari, tidak menunjukkan resistan terhadap rawatan dengan cepat. Secara kolektif, kajian ini menunjukan bahawa Maritoclax sebagai ejen tunggal atau gabungan dengan BH3 mimetik boleh menjadi strategi rawatan yang berpotensi untuk NPC.
Keywords: 3D NPC Spheroids; ABT-263; BH3 Mimetics; Maritoclax; Nasopharyngeal Carcinoma.
© Penerbit Universiti Sains Malaysia, 2020.
Figures


References
-
- Airiau K, Prouzet-Mauleon V, Rousseau B, Pigneux A, Jeanneteau M, Giraudon M, Allou K, Dubus P, Belloc F, Mahon FX. Synergistic cooperation between ABT-263 and MEK1/2 inhibitor: effect on apoptosis and proliferation of acute myeloid leukemia cells. Oncotarget. 2016;7(1):845–859. doi: 10.18632/oncotarget.6417. - DOI - PMC - PubMed
-
- Allen JD, Jackson SC, Schinkel AH. A mutation hot spot in the Bcrp1 (Abcg2) multidrug transporter in mouse cell lines selected for Doxorubicin resistance. Cancer Research. 2002;62(8):2294–2299. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
