The Role of Metallic Nanoparticles in Inhibition of Mycobacterium Tuberculosis and Enhances Phagosome Maturation into the Infected Macrophage
- PMID: 33214909
- PMCID: PMC7658918
- DOI: 10.5001/omj.2020.78
The Role of Metallic Nanoparticles in Inhibition of Mycobacterium Tuberculosis and Enhances Phagosome Maturation into the Infected Macrophage
Abstract
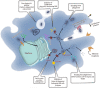
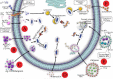
This review focuses on the role of gallium (Ga) nanoparticles (NPs) to enhance phagosome maturation into the Mycobacterium tuberculosis-infected macrophage and the role of magnetic iron NPs as nanocarriers of antituberculosis drugs. The literature shows that silver (Ag) and zinc oxide (ZnO) NPs with dimensions less than 10 nm can penetrate directly through the macrophage bilayer membrane. Ag NPs increase the permeability membrane by motiving the aggregation of proteins in the periplasmic space and forming nano-sized pores. ZnO NPs can interact with the membrane of M. tuberculosis, which leads to the formation of surface pores and the release of intracellular nucleotides. The colloidal Ag:ZnO mixture NPs with 1:1 ratio can eliminate M. tuberculosis and shows the lowest cytotoxicity effects on MCF-7 and THP-1 cell lines. Ag/ZnO nanocrystals are not able to kill M. tuberculosis alone ex-vivo. Hence, bimetallic gold (Au)/Ag NPs possessed high efficiency to inhibit M. tuberculosis in an ex-vivo THP-1 infection model. Co-delivery of mixed MeNPs into a polymeric carrier collaborated to selective uptake by macrophages through passive targeting, initial burst release of ions from the encapsulated metallic (Me) NPs, and eventually, reduction of MeNPs toxicity, and plays a pivotal role in increasing the antitubercular activity compared to use alone. In addition, Ga NPs can import drugs to the macrophage, inhibit M. tuberculosis growth, and reduce the inhibition of phagosome maturation. Magnetic encapsulated NPs exhibited good drug release properties and might be suitable as carriers of antituberculosis drugs.
Keywords: Macrophages; Metal Nanoparticles; Mycobacterium tuberculosis; Pharmaceutical Preparations.
The OMJ is Published Bimonthly and Copyrighted 2020 by the OMSB.
Figures

Similar articles
-
Mixed metal oxide nanoparticles inhibit growth of Mycobacterium tuberculosis into THP-1 cells.Int J Mycobacteriol. 2016 Dec;5 Suppl 1:S181-S183. doi: 10.1016/j.ijmyco.2016.09.011. Epub 2016 Nov 5. Int J Mycobacteriol. 2016. PMID: 28043541
-
Bactericidal impact of Ag, ZnO and mixed AgZnO colloidal nanoparticles on H37Rv Mycobacterium tuberculosis phagocytized by THP-1 cell lines.Microb Pathog. 2017 Sep;110:335-344. doi: 10.1016/j.micpath.2017.07.010. Epub 2017 Jul 11. Microb Pathog. 2017. PMID: 28710015
-
Multimetallic Microparticles Increase the Potency of Rifampicin against Intracellular Mycobacterium tuberculosis.ACS Nano. 2018 Jun 26;12(6):5228-5240. doi: 10.1021/acsnano.7b08264. Epub 2018 May 22. ACS Nano. 2018. PMID: 29767993
-
Nanotechnology as a therapeutic tool to combat microbial resistance.Adv Drug Deliv Rev. 2013 Nov;65(13-14):1803-15. doi: 10.1016/j.addr.2013.07.011. Epub 2013 Jul 24. Adv Drug Deliv Rev. 2013. PMID: 23892192 Review.
-
Mycobacteria, metals, and the macrophage.Immunol Rev. 2015 Mar;264(1):249-63. doi: 10.1111/imr.12265. Immunol Rev. 2015. PMID: 25703564 Free PMC article. Review.
Cited by
-
Antioxidants: Classification, Natural Sources, Activity/Capacity Measurements, and Usefulness for the Synthesis of Nanoparticles.Materials (Basel). 2021 Jul 25;14(15):4135. doi: 10.3390/ma14154135. Materials (Basel). 2021. PMID: 34361329 Free PMC article. Review.
-
Photothermal therapy of tuberculosis using targeting pre-activated macrophage membrane-coated nanoparticles.Nat Nanotechnol. 2024 Jun;19(6):834-845. doi: 10.1038/s41565-024-01618-0. Epub 2024 Feb 21. Nat Nanotechnol. 2024. PMID: 38383890
-
Impact of nanoparticles on amyloid β-induced Alzheimer's disease, tuberculosis, leprosy and cancer: a systematic review.Biosci Rep. 2023 Feb 27;43(2):BSR20220324. doi: 10.1042/BSR20220324. Biosci Rep. 2023. PMID: 36630532 Free PMC article.
-
In vivo antimicrobial activity of engineered mesoporous silica nanoparticles targeting intracellular mycobacteria.Nat Commun. 2025 Aug 11;16(1):7388. doi: 10.1038/s41467-025-62623-y. Nat Commun. 2025. PMID: 40790109 Free PMC article.
-
From infection niche to therapeutic target: the intracellular lifestyle of Mycobacterium tuberculosis.Microbiology (Reading). 2021 Apr;167(4):001041. doi: 10.1099/mic.0.001041. Microbiology (Reading). 2021. PMID: 33826491 Free PMC article. Review.
References
-
- World Health Organization. Global tuberculosis control: epidemiology, strategy, financing: WHO report 2009; 2009.
Publication types
LinkOut - more resources
Full Text Sources
