Study of Glucose-6-Phosphate Dehydrogenase (G6PD) Deficiency and Genotype Polymorphism of G6PD B and G6PD (A+/A-) in Patients Treated for Plasmodium vivax Malaria in a Tertiary Care Hospital in North East India
- PMID: 33214970
- PMCID: PMC7671175
- DOI: 10.7759/cureus.11463
Study of Glucose-6-Phosphate Dehydrogenase (G6PD) Deficiency and Genotype Polymorphism of G6PD B and G6PD (A+/A-) in Patients Treated for Plasmodium vivax Malaria in a Tertiary Care Hospital in North East India
Abstract
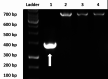
Introduction Glucose-6-phosphate dehydrogenase (G6PD) enzyme deficiency is the most common enzymopathy in humans, and its distribution has been historically described to be closely associated with that of malaria. North East India provides optimal conditions for transmission of malaria and bears a considerable burden of Plasmodium vivax (P. vivax) malaria. Primaquine, a mainstay in the treatment of vivax malaria, may trigger episodes of acute hemolysis in patients with G6PD deficiency. The present study sought to delineate the frequency and genotypes of G6PD deficiency among patients suffering from vivax malaria infections. Methods Blood specimens from 80 individuals diagnosed with vivax malaria underwent enzyme assay for G6PD deficiency. Samples with deficient phenotype underwent isolation of DNA using a genomic DNA isolation kit (Qiagen India Pvt. Ltd., New Delhi, India). The genomic DNA underwent amplification, serial denaturation, annealing, extension, final extension followed by digestion with restriction endonucleases Nla III and Fok I. The digested products were subjected to horizontal agarose electrophoresis for the separation of digested fragments. Samples without nucleotide 376 adenine→guanine (A→G) mutation were classified as G6PD B. Those with the mutation were further classified into G6PD A(+) and G6PD A(-) based on the presence of Nla III site. Results Twenty-seven out of 80 individuals (33.75%) with P. vivax malaria were found to have G6PD deficiency, of which a majority (n=24) had G6PD B genotype. Three individuals had Asparagine→Aspartic Acid mutation at position 376 (A→G), of which G6PD A(+) and G6PD A(-) were present in two and one cases, respectively. Conclusion G6PD deficiency was noted in about a third of patients with vivax malaria. Since primaquine therapy is contraindicated in this group of patients, there is a rationale for looking into screening patients with vivax malaria from the region prior to primaquine therapy. Further large scale studies may substantiate this and help in better genotypic and geographic characterization of G6PD deficiency in the region.
Keywords: g6pd a(+); g6pd a(-); g6pd b; g6pd genotype; glucose-6-phosphate-dehydrogenase deficiency (g6pd); malaria; north east india; plasmodium vivax.
Copyright © 2020, Rajkhowa et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Prevalence of G6PD Viangchan variant in malaria endemic areas in Lao PDR: an implication for malaria elimination by 2030.Malar J. 2019 Mar 12;18(1):75. doi: 10.1186/s12936-019-2715-0. Malar J. 2019. PMID: 30866940 Free PMC article.
-
[Role of primaquine in malaria control and elimination in French-speaking Africa].Bull Soc Pathol Exot. 2017 Aug;110(3):198-206. doi: 10.1007/s13149-017-0556-z. Epub 2017 Apr 17. Bull Soc Pathol Exot. 2017. PMID: 28417346 Review. French.
-
Primaquine alternative dosing schedules for preventing malaria relapse in people with Plasmodium vivax.Cochrane Database Syst Rev. 2020 Aug 19;8:CD012656. doi: 10.1002/14651858.CD012656.pub3. Cochrane Database Syst Rev. 2020. PMID: 32816320
-
The impact of phenotypic and genotypic G6PD deficiency on risk of plasmodium vivax infection: a case-control study amongst Afghan refugees in Pakistan.PLoS Med. 2010 May 25;7(5):e1000283. doi: 10.1371/journal.pmed.1000283. PLoS Med. 2010. PMID: 20520804 Free PMC article.
-
Investigation of glucose-6-phosphate dehydrogenase (G6PD) deficiency prevalence in a Plasmodium vivax-endemic area in the Republic of Korea (ROK).Malar J. 2020 Sep 1;19(1):317. doi: 10.1186/s12936-020-03393-4. Malar J. 2020. PMID: 32873296 Free PMC article.
Cited by
-
Computational analysis of dimer G6PD structure to elucidate pathogenicity of G6PD variants.Biomedicine (Taipei). 2024 Mar 1;14(1):47-59. doi: 10.37796/2211-8039.1431. eCollection 2024. Biomedicine (Taipei). 2024. PMID: 38533298 Free PMC article.
-
The First Report of Three Glucose-6-Phosphate Dehydrogenase (G6PD) Variants: Mediterranean, Orissa and Kalyan-Kerala from Northeast India.Indian J Hematol Blood Transfus. 2024 Jan;40(1):175-176. doi: 10.1007/s12288-023-01686-7. Epub 2023 Aug 10. Indian J Hematol Blood Transfus. 2024. PMID: 38312194 Free PMC article. No abstract available.
-
Genotypic glucose-6-phosphate dehydrogenase (G6PD) deficiency protects against Plasmodium falciparum infection in individuals living in Ghana.PLoS One. 2021 Sep 27;16(9):e0257562. doi: 10.1371/journal.pone.0257562. eCollection 2021. PLoS One. 2021. PMID: 34570821 Free PMC article.
References
-
- Haplotype diversity and linkage disequilibrium at human G6PD: recent origin of alleles that confer malarial resistance. Tishkoff SA, Varkonyi R, Cahinhinan N, et al. Science. 2001;293:455–462. - PubMed
-
- G6PD deficiency: the genotype-phenotype association. Mason PJ, Bautista JM, Gilsanz F. Blood Rev. 2007;21:267–283. - PubMed
-
- Sequence of human glucose-6-phosphate dehydrogenase cloned in plasmids and a yeast artificial chromosome. Chen EY, Cheng A, Lee A, et al. Genomics. 1991;10:792–800. - PubMed
-
- Human glucose-6-phosphate dehydrogenase: purification of the erythrocyte enzyme and the influence of ions on its activity. Cohen P, Rosemeyer MA. Eur J Biochem. 1969;8:1–7. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
