COVID-19 antibody therapeutics tracker: a global online database of antibody therapeutics for the prevention and treatment of COVID-19
- PMID: 33215063
- PMCID: PMC7454247
- DOI: 10.1093/abt/tbaa020
COVID-19 antibody therapeutics tracker: a global online database of antibody therapeutics for the prevention and treatment of COVID-19
Abstract
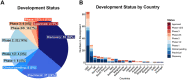
Facing the COVID-19 global healthcare crisis, scientists worldwide are collaborating to develop prophylactic and therapeutic interventions against the disease. Antibody therapeutics hold enormous promise for the treatment of COVID-19. In March 2020, the Chinese Antibody Society, in collaboration with The Antibody Society, initiated the "COVID-19 Antibody Therapeutics Tracker" ("Tracker") (https://chineseantibody.org/covid-19-track/) program to track the antibody-based COVID-19 interventions in preclinical and clinical development globally. The data are collected from the public domain and verified by volunteers on an ongoing basis. Here, we present exploratory data analyses and visualization to demonstrate the latest trends of COVID-19 antibody development, based on data for over 150 research and development programs and molecules included in the "Tracker" as of 8 August 2020. We categorized the data mainly by their targets, formats, development status, developers and country of origin. Although details are limited in some cases, all of the anti-SARS-CoV-2 antibody candidates appear to target the viral spike protein (S protein), and most are full-length monoclonal antibodies. Most of the current COVID-19 antibody therapeutic candidates in clinical trials are repurposed drugs aimed at targets other than virus-specific proteins, while most of these virus-specific therapeutic antibodies are in discovery or preclinical studies. As of 8 August 2020, eight antibody candidates targeting the SARS-CoV-2 S protein have entered clinical studies, including LY-CoV555, REGN-COV2, JS016, TY027, CT-P59, BRII-196, BRII-198 and SCTA01. Ongoing clinical trials of SARS-CoV-2 neutralizing antibodies will help define the utility of these antibodies as a new class of therapeutics for treating COVID-19 and future coronavirus infections.
Keywords: COVID-19; SARS-CoV-2; clinical trial; neutralizing antibody; online database; spike (S) protein.
Published by Oxford University Press on behalf of Antibody Therapeutics 2020.
Figures

References
-
- Huser, V, Cimino, JJ. Linking ClinicalTrials.Gov and PubMed to track results of interventional human clinical trials. PLoS One 2013; 8: e68409. - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
