The effect of spironolactone on cardiovascular function and markers of fibrosis in people at increased risk of developing heart failure: the heart 'OMics' in AGEing (HOMAGE) randomized clinical trial
- PMID: 33215209
- PMCID: PMC7878013
- DOI: 10.1093/eurheartj/ehaa758
The effect of spironolactone on cardiovascular function and markers of fibrosis in people at increased risk of developing heart failure: the heart 'OMics' in AGEing (HOMAGE) randomized clinical trial
Abstract
Aims: To investigate the effects of spironolactone on fibrosis and cardiac function in people at increased risk of developing heart failure.
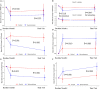
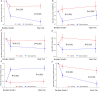
Methods and results: Randomized, open-label, blinded-endpoint trial comparing spironolactone (50 mg/day) or control for up to 9 months in people with, or at high risk of, coronary disease and raised plasma B-type natriuretic peptides. The primary endpoint was the interaction between baseline serum galectin-3 and changes in serum procollagen type-III N-terminal pro-peptide (PIIINP) in participants assigned to spironolactone or control. Procollagen type-I C-terminal pro-peptide (PICP) and collagen type-1 C-terminal telopeptide (CITP), reflecting synthesis and degradation of type-I collagen, were also measured. In 527 participants (median age 73 years, 26% women), changes in PIIINP were similar for spironolactone and control [mean difference (mdiff): -0.15; 95% confidence interval (CI) -0.44 to 0.15 μg/L; P = 0.32] but those receiving spironolactone had greater reductions in PICP (mdiff: -8.1; 95% CI -11.9 to -4.3 μg/L; P < 0.0001) and PICP/CITP ratio (mdiff: -2.9; 95% CI -4.3 to -1.5; <0.0001). No interactions with serum galectin were observed. Systolic blood pressure (mdiff: -10; 95% CI -13 to -7 mmHg; P < 0.0001), left atrial volume (mdiff: -1; 95% CI -2 to 0 mL/m2; P = 0.010), and NT-proBNP (mdiff: -57; 95% CI -81 to -33 ng/L; P < 0.0001) were reduced in those assigned spironolactone.
Conclusions: Galectin-3 did not identify greater reductions in serum concentrations of collagen biomarkers in response to spironolactone. However, spironolactone may influence type-I collagen metabolism. Whether spironolactone can delay or prevent progression to symptomatic heart failure should be investigated.
Keywords: Collagen markers; Fibrosis; Heart failure prevention; Spironolactone.
© The Author(s) 2020. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures


Comment in
-
Detection of patients at risk of developing heart failure responsive to mineralocorticoid receptor antagonists (MRAs): new insights and opportunities.Eur Heart J. 2021 Feb 11;42(6):697-699. doi: 10.1093/eurheartj/ehaa765. Eur Heart J. 2021. PMID: 33257941 No abstract available.
References
-
- Zannad F. Rising incidence of heart failure demands action. Lancet 2018;391:518–519. - PubMed
-
- Cleland JGF, Pellicori P, Clark AL. Prevention or procrastination for heart failure? Why we need a universal definition of heart failure. J Am Coll Cardiol 2019;73:2398–2400. - PubMed
-
- Ferreira JP, Verdonschot J, Collier T, Wang P, Pizard A, Bar C, Bjorkman J, Boccanelli A, Butler J, Clark A, Cleland JG, Delles C, Diez J, Girerd N, Gonzalez A, Hazebroek M, Huby AC, Jukema W, Latini R, Leenders J, Levy D, Mebazaa A, Mischak H, Pinet F, Rossignol P, Sattar N, Sever P, Staessen JA, Thum T, Vodovar N, Zhang ZY, Heymans S, Zannad F. Proteomic bioprofiles and mechanistic pathways of progression to heart failure. Circ Heart Fail 2019;12:e005897. - PMC - PubMed
-
- Tromp J, Westenbrink BD, Ouwerkerk W, Van Veldhuisen DJ, Samani NJ, Ponikowski P, Metra M, Anker SD, Cleland JG, Dickstein K, Filippatos G, van der Harst P, Lang CC, Ng LL, Zannad F, Zwinderman AH, Hillege HL, van der Meer P, Voors AA. Identifying pathophysiological mechanisms in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol 2018;72:1081–1090. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

