S100a8 silencing attenuates inflammation, oxidative stress and apoptosis in BV2 cells induced by oxygen‑glucose deprivation and reoxygenation by upregulating GAB1 expression
- PMID: 33215218
- PMCID: PMC7716398
- DOI: 10.3892/mmr.2020.11702
S100a8 silencing attenuates inflammation, oxidative stress and apoptosis in BV2 cells induced by oxygen‑glucose deprivation and reoxygenation by upregulating GAB1 expression
Abstract
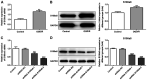
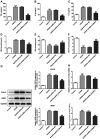
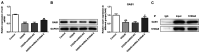
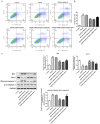
S100a8 serves an important role in cell differentiation and is abnormally expressed in common tumors, but there are few studies on the association between S100a8 and brain I/R injury. The present study aimed to investigate the role of S100a8 in oxygen‑glucose deprivation and reoxygenation (OGD/R)‑induced BV2 microglia cell injury, and to elucidate the potential underlying molecular mechanisms. BV2 cells were exposed to OGD/R to mimic ischemia/reperfusion (I/R) injury in vitro. S100a8 expression was detected via reverse transcription‑quantitative PCR and western blot analyses. Following transfection with short hairpin RNAs targeting S100a8, the levels of inflammatory cytokines and oxidative stress‑related factors were determined using commercial kits. Apoptosis was assessed using flow cytometric analysis and the expression levels of apoptosis‑related proteins were determined using western blot analysis. Subsequently, the mRNA and protein levels of Grb2‑associated binder 1 (GAB1) were assessed following S100a8 silencing. Immunoprecipitation (IP) was performed to verify the association between S100a8 and GAB1. The levels of inflammation, oxidative stress and apoptosis were assessed following GAB1 silencing, along with S100a8 silencing in BV2 cells subjected to OGD/R. The results indicated that exposure to OGD/R markedly upregulated S100a8 expression in BV2 cells. S100a8 silencing inhibited inflammation, oxidative stress and apoptosis, accompanied by changes in the expression of related proteins. The IP assay revealed a strong interaction between GAB1 and S100a8. In addition, GAB1 silencing reversed the inhibitory effects of S100a8 silencing on inflammation, oxidative stress and apoptosis in OGD/R‑stimulated BV2 cells. Taken together, the results of the present study demonstrated that S100a8 silencing alleviated inflammation, oxidative stress and the apoptosis of BV2 cells induced by OGD/R, partly by upregulating the expression of GAB1. Thus, these findings may potentially provide a novel direction to develop therapeutic strategies for cerebral I/R injury.
Keywords: inflammation; apoptosis; oxygen‑glucose deprivation; S100a8; Grb2‑associated binder 1.
Figures


References
-
- Howlett JA, Northington FJ, Gilmore MM, Tekes A, Huisman TA, Parkinson C, Chung SE, Jennings JM, Jamrogowicz JJ, Larson AC, et al. Cerebrovascular autoregulation and neurologic injury in neonatal hypoxic-ischemic encephalopathy. Pediatr Res. 2013;74:525–535. doi: 10.1038/pr.2013.132. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

