The impact of demographic, clinical, genetic, and imaging variables on tau PET status
- PMID: 33215319
- PMCID: PMC8131404
- DOI: 10.1007/s00259-020-05099-w
The impact of demographic, clinical, genetic, and imaging variables on tau PET status
Abstract
Purpose: A substantial proportion of amyloid-β (Aβ)+ patients with clinically diagnosed Alzheimer's disease (AD) dementia and mild cognitive impairment (MCI) are tau PET-negative, while some clinically diagnosed non-AD neurodegenerative disorder (non-AD) patients or cognitively unimpaired (CU) subjects are tau PET-positive. We investigated which demographic, clinical, genetic, and imaging variables contributed to tau PET status.
Methods: We included 2338 participants (430 Aβ+ AD dementia, 381 Aβ+ MCI, 370 non-AD, and 1157 CU) who underwent [18F]flortaucipir (n = 1944) or [18F]RO948 (n = 719) PET. Tau PET positivity was determined in the entorhinal cortex, temporal meta-ROI, and Braak V-VI regions using previously established cutoffs. We performed bivariate binary logistic regression models with tau PET status (positive/negative) as dependent variable and age, sex, APOEε4, Aβ status (only in CU and non-AD analyses), MMSE, global white matter hyperintensities (WMH), and AD-signature cortical thickness as predictors. Additionally, we performed multivariable binary logistic regression models to account for all other predictors in the same model.
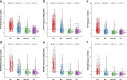
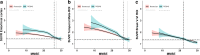
Results: Tau PET positivity in the temporal meta-ROI was 88.6% for AD dementia, 46.5% for MCI, 9.5% for non-AD, and 6.1% for CU. Among Aβ+ participants with AD dementia and MCI, lower age, MMSE score, and AD-signature cortical thickness showed the strongest associations with tau PET positivity. In non-AD and CU participants, presence of Aβ was the strongest predictor of a positive tau PET scan.
Conclusion: We identified several demographic, clinical, and neurobiological factors that are important to explain the variance in tau PET retention observed across the AD pathological continuum, non-AD neurodegenerative disorders, and cognitively unimpaired persons.
Keywords: Alzheimer’s disease; Aβ; Dementia; MCI; PET; Tau.
Conflict of interest statement
OH has acquired research support (for the institution) from Roche, Pfizer, GE Healthcare, Biogen, Eli Lilly, and AVID Radiopharmaceuticals. In the past 2 years, he has received consultancy/speaker fees (paid to the institution) from Biogen and Roche.
GDR receives research support from NIH, Alz Assoc, American College of Radiology, Avid Radiopharmaceuticals, GE Healthcare, Life Molecular Imaging. In the past 2 years he has received consulting fees from Axon Neurosciences, Eisai, GE Healthcare, Johnson & Johnson, and Merck. He is an Associate Editor for JAMA Neurology.
ALB receives research support from NIH (R01AG038791, U19AG063911), the Tau Research Consortium, the Association for Frontotemporal Degeneration, and the Bluefield Project to Cure Frontotemporal Dementia. He has served as a consultant for AGTC, Alector, Arkuda, Arvinas, Bioage, Ionis, Lundbeck, Passage BIO, Samumed, Ono, Sangamo, Stealth, Third Rock, Transposon, UCB, and Wave, and received research support from Avid, Eisai, Biogen, and Roche.
MJP and MDD are employees of Avid Radiopharmaceuticals, a wholly owned subsidiary of Eli Lilly and Company, and are minor stockholders in Eli Lilly.
EB and GK are employees of F. Hoffmann-La Roche Ltd.
The other authors report no conflict of interest.
Figures
References
-
- US Food & Drug administration. https://www.fda.gov/news-events/press-announcements/fda-approves-first-d.... Accessed 28 May 2020.

