Plasminogen Deficiency and Amiloride Mitigate Angiotensin II-Induced Hypertension in Type 1 Diabetic Mice Suggesting Effects Through the Epithelial Sodium Channel
- PMID: 33215566
- PMCID: PMC7763785
- DOI: 10.1161/JAHA.120.016387
Plasminogen Deficiency and Amiloride Mitigate Angiotensin II-Induced Hypertension in Type 1 Diabetic Mice Suggesting Effects Through the Epithelial Sodium Channel
Abstract
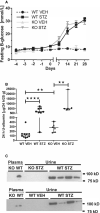
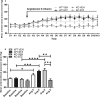
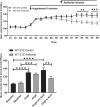
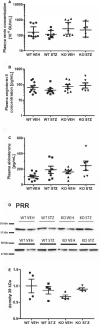
Background Diabetic nephropathy is a common diabetes mellitus complication associated with hypertension, proteinuria, and excretion of urinary plasmin that activates the epithelial sodium channel, ENaC, in vitro. Here we hypothesized that the deletion of plasminogen and amiloride treatment protect against hypertension in diabetes mellitus. Methods and Results Male plasminogen knockout (plasminogen-deficient [Plg-/-]) and wild-type mice were rendered diabetic with streptozotocin. Arterial blood pressure was recorded continuously by indwelling catheters before and during 10 days of angiotensin II infusion (ANGII; 30-60 ng/kg per minute). The effect of amiloride infusion (2 mg/kg per day, 4 days) was tested in wild-type, diabetic ANGII-treated mice. Streptozotocin increased plasma and urine glucose concentrations and 24-hour urine albumin and plasminogen excretion. Diabetic Plg-/- mice displayed larger baseline albuminuria and absence of urine plasminogen. Baseline mean arterial blood pressure did not differ between groups. Although ANGII elevated blood pressure in wild-type, diabetic wild-type, and Plg-/- control mice, ANGII did not change blood pressure in diabetic Plg-/- mice. Compared with ANGII infusion alone, wild-type ANGII-infused diabetic mice showed blood pressure reduction upon amiloride treatment. There was no difference in plasma renin, ANGII, aldosterone, tissue prorenin receptor, renal inflammation, and fibrosis between groups. Urine from wild-type mice evoked larger amiloride-sensitive current than urine from Plg-/- mice with or without diabetes mellitus. Full-length γ-ENaC and α-ENaC subunit abundances were not changed in kidney homogenates, but the 70 kDa γ-ENaC cleavage product was increased in diabetic versus nondiabetic mice. Conclusions Plasmin promotes hypertension in diabetes mellitus with albuminuria likely through the epithelial sodium channel.
Keywords: albuminuria; aldosterone; protease; renin; sodium channels.
Conflict of interest statement
Dr Hansen is an AstraZeneca employee and holds stocks in the company. The remaining authors have no disclosures to report.
Figures

Similar articles
-
Significant natriuretic and antihypertensive action of the epithelial sodium channel blocker amiloride in diabetic patients with and without nephropathy.J Hypertens. 2016 Aug;34(8):1621-9. doi: 10.1097/HJH.0000000000000967. J Hypertens. 2016. PMID: 27214087
-
Amiloride lowers blood pressure and attenuates urine plasminogen activation in patients with treatment-resistant hypertension.J Am Soc Hypertens. 2014 Dec;8(12):872-81. doi: 10.1016/j.jash.2014.09.019. J Am Soc Hypertens. 2014. PMID: 25492830 Clinical Trial.
-
Amiloride evokes significant natriuresis and weight loss in kidney transplant recipients with and without albuminuria.Am J Physiol Renal Physiol. 2023 Oct 1;325(4):F426-F435. doi: 10.1152/ajprenal.00108.2023. Epub 2023 Aug 10. Am J Physiol Renal Physiol. 2023. PMID: 37560772 Clinical Trial.
-
Urinary serine proteases and activation of ENaC in kidney--implications for physiological renal salt handling and hypertensive disorders with albuminuria.Pflugers Arch. 2015 Mar;467(3):531-42. doi: 10.1007/s00424-014-1661-5. Epub 2014 Dec 9. Pflugers Arch. 2015. PMID: 25482671 Review.
-
Mechanisms of renal NaCl retention in proteinuric disease.Acta Physiol (Oxf). 2013 Mar;207(3):536-45. doi: 10.1111/apha.12047. Epub 2013 Jan 7. Acta Physiol (Oxf). 2013. PMID: 23216619 Review.
Cited by
-
A Serine Protease Inhibitor, Camostat Mesilate, Suppresses Urinary Plasmin Activity and Alleviates Hypertension and Podocyte Injury in Dahl Salt-Sensitive Rats.Int J Mol Sci. 2023 Oct 30;24(21):15743. doi: 10.3390/ijms242115743. Int J Mol Sci. 2023. PMID: 37958726 Free PMC article.
-
Beyond Blood Pressure: Emerging Pathways and Precision Approaches in Hypertension-Induced Kidney Damage.Int J Mol Sci. 2025 Aug 6;26(15):7606. doi: 10.3390/ijms26157606. Int J Mol Sci. 2025. PMID: 40806733 Free PMC article. Review.
-
Amiloride Reduces Urokinase/Plasminogen-Driven Intratubular Complement Activation in Glomerular Proteinuria.J Am Soc Nephrol. 2024 Apr 1;35(4):410-425. doi: 10.1681/ASN.0000000000000312. Epub 2024 Jan 23. J Am Soc Nephrol. 2024. PMID: 38254266 Free PMC article.
-
Interleukin 17A infusion has no acute or long-term hypertensive action in conscious unrestrained male mice.Pflugers Arch. 2022 Jul;474(7):709-719. doi: 10.1007/s00424-022-02705-8. Epub 2022 May 23. Pflugers Arch. 2022. PMID: 35604452
-
The Epithelial Sodium Channel-An Underestimated Drug Target.Int J Mol Sci. 2023 Apr 24;24(9):7775. doi: 10.3390/ijms24097775. Int J Mol Sci. 2023. PMID: 37175488 Free PMC article. Review.
References
-
- Krolewski AS, Warram JH, Christlieb AR, Busick EJ, Kahn CR. The changing natural history of nephropathy in type I diabetes. Am J Med. 1985;78:785–794. - PubMed
-
- Parving H‐H, Persson F, Rossing P. Microalbuminuria: a parameter that has changed diabetes care. Diabetes Res Clin Pract. 2015;107:1–8. - PubMed
-
- Strojek K, Grzeszczak W, Lacka B, Gorska J, Keller C, Ritz E. Increased prevalence of salt sensitivity of blood pressure in IDDM with and without microalbuminuria. Diabetologia. 1995;38:1443–1448. - PubMed
-
- Bigazzi R, Bianchi S, Baldari D, Sgherri G, Baldari G, Campese VM. Microalbuminuria in salt‐sensitive patients. A marker for renal and cardiovascular risk factors. Hypertension. 1994;23:195–199. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

