Tumor Frameshift Mutation Proportion Predicts Response to Immunotherapy in Mismatch Repair-Deficient Prostate Cancer
- PMID: 33215787
- PMCID: PMC7873327
- DOI: 10.1002/onco.13601
Tumor Frameshift Mutation Proportion Predicts Response to Immunotherapy in Mismatch Repair-Deficient Prostate Cancer
Abstract
Background: Genomic biomarkers that predict response to anti-PD1 therapy in prostate cancer are needed. Frameshift mutations are predicted to generate more neoantigens than missense mutations; therefore, we hypothesized that the number or proportion of tumor frameshift mutations would correlate with response to anti-PD1 therapy in prostate cancer.
Methods: To enrich for response to anti-PD1 therapy, we assembled a multicenter cohort of 65 men with mismatch repair-deficient (dMMR) prostate cancer. Patient characteristics and outcomes were determined by retrospective chart review. Clinical somatic DNA sequencing was used to determine tumor mutational burden (TMB), frameshift mutation burden, and frameshift mutation proportion (FSP), which were correlated to outcomes on anti-PD1 treatment. We subsequently used data from a clinical trial of pembrolizumab in patients with nonprostatic dMMR cancers of various histologies as a biomarker validation cohort.
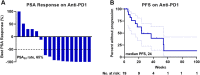
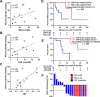
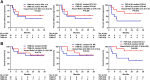
Results: Nineteen of 65 patients with dMMR metastatic castration-resistant prostate cancer were treated with anti-PD1 therapy. The PSA50 response rate was 65%, and the median progression-free survival (PFS) was 24 (95% confidence interval 16-54) weeks. Tumor FSP, more than overall TMB, correlated most strongly with prolonged PFS and overall survival (OS) on anti-PD1 treatment and with density of CD8+ tumor-infiltrating lymphocytes. High FSP similarly identified patients with longer PFS as well as OS on anti-PD1 therapy in a validation cohort.
Conclusion: Tumor FSP correlated with prolonged efficacy of anti-PD1 treatment among patients with dMMR cancers and may represent a new biomarker of immune checkpoint inhibitor sensitivity.
Implications for practice: Given the modest efficacy of immune checkpoint inhibition (ICI) in unselected patients with advanced prostate cancer, biomarkers of ICI sensitivity are needed. To facilitate biomarker discovery, a cohort of patients with DNA mismatch repair-deficient (dMMR) prostate cancer was assembled, as these patients are enriched for responses to ICI. A high response rate to anti-PD1 therapy in these patients was observed; however, these responses were not durable in most patients. Notably, tumor frameshift mutation proportion (FSP) was identified as a novel biomarker that was associated with prolonged response to anti-PD1 therapy in this cohort. This finding was validated in a separate cohort of patients with nonprostatic dMMR cancers of various primary histologies. This works suggests that FSP predicts response to anti-PD1 therapy in dMMR cancers, which should be validated prospectively in larger independent cohorts.
Keywords: Biomarkers; Immunotherapy; Mismatch repair deficiency; Prostate cancer.
© 2020 The Authors. The Oncologist published by Wiley Periodicals LLC on behalf of AlphaMed Press.
Conflict of interest statement
Figures


References
-
- Beer TM, Kwon ED, Drake CG et al. Randomized, double‐blind, phase III trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy‐naive castration‐resistant prostate cancer. J Clin Oncol 2017;35:40–47. - PubMed
-
- Kwon ED, Drake CG, Scher HI et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration‐resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184‐043): A multicentre, randomised, double‐blind, phase 3 trial. Lancet Oncol 2014;15:700–712. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

