Competition between Usutu virus and West Nile virus during simultaneous and sequential infection of Culex pipiens mosquitoes
- PMID: 33215969
- PMCID: PMC7738303
- DOI: 10.1080/22221751.2020.1854623
Competition between Usutu virus and West Nile virus during simultaneous and sequential infection of Culex pipiens mosquitoes
Abstract
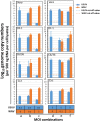
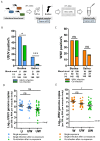
Usutu virus (USUV) and West Nile virus (WNV) are closely related mosquito-borne flaviviruses that are mainly transmitted between bird hosts by vector mosquitoes. Infections in humans are incidental but can cause severe disease. USUV is endemic in large parts of Europe, while WNV mainly circulates in Southern Europe. In recent years, WNV is also frequently detected in Northern Europe, thereby expanding the area where both viruses co-circulate. However, it remains unclear how USUV may affect the future spread of WNV and the likelihood of human co-infection. Here we investigated whether co-infections with both viruses in cell lines and their primary mosquito vector, Culex pipiens, affect virus replication and transmission dynamics. We show that USUV is outcompeted by WNV in mammalian, avian and mosquito cells during co-infection. Mosquitoes that were exposed to both viruses simultaneously via infectious blood meal displayed significantly reduced USUV transmission compared to mosquitoes that were only exposed to USUV (from 15% to 3%), while the infection and transmission of WNV was unaffected. In contrast, when mosquitoes were pre-infected with USUV via infectious blood meal, WNV transmission was significantly reduced (from 44% to 17%). Injection experiments established the involvement of the midgut in the observed USUV-mediated WNV inhibition. The competition between USUV and WNV during co-infection clearly indicates that the chance of concurrent USUV and WNV transmission via a single mosquito bite is low. The competitive relation between USUV and WNV may impact virus transmission dynamics in the field and affect the epidemiology of WNV in Europe.
Keywords: Usutu virus; West Nile virus; competition; mosquito; vector competence.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



Similar articles
-
Simultaneous Coinfections with West Nile Virus and Usutu Virus in Culex pipiens and Aedes vexans Mosquitoes.Transbound Emerg Dis. 2023 Mar 29;2023:6305484. doi: 10.1155/2023/6305484. eCollection 2023. Transbound Emerg Dis. 2023. PMID: 40303695 Free PMC article.
-
Noncoding Subgenomic Flavivirus RNA Is Processed by the Mosquito RNA Interference Machinery and Determines West Nile Virus Transmission by Culex pipiens Mosquitoes.J Virol. 2016 Oct 28;90(22):10145-10159. doi: 10.1128/JVI.00930-16. Print 2016 Nov 15. J Virol. 2016. PMID: 27581979 Free PMC article.
-
West Nile and Usutu viruses co-circulation in central Italy: outcomes of the 2018 integrated surveillance.Parasit Vectors. 2021 May 7;14(1):243. doi: 10.1186/s13071-021-04736-z. Parasit Vectors. 2021. PMID: 33962673 Free PMC article.
-
Usutu Virus: An Arbovirus on the Rise.Viruses. 2019 Jul 12;11(7):640. doi: 10.3390/v11070640. Viruses. 2019. PMID: 31336826 Free PMC article. Review.
-
Mosquito species involved in the circulation of West Nile and Usutu viruses in Italy.Vet Ital. 2017 Jun 30;53(2):97-110. doi: 10.12834/VetIt.114.933.4764.2. Vet Ital. 2017. PMID: 28675249 Review.
Cited by
-
Simultaneous Coinfections with West Nile Virus and Usutu Virus in Culex pipiens and Aedes vexans Mosquitoes.Transbound Emerg Dis. 2023 Mar 29;2023:6305484. doi: 10.1155/2023/6305484. eCollection 2023. Transbound Emerg Dis. 2023. PMID: 40303695 Free PMC article.
-
North American House Sparrows Are Competent for Usutu Virus Transmission.mSphere. 2022 Dec 21;7(6):e0029522. doi: 10.1128/msphere.00295-22. Epub 2022 Nov 1. mSphere. 2022. PMID: 36317895 Free PMC article.
-
Belgian Culex pipiens pipiens are competent vectors for West Nile virus while Culex modestus are competent vectors for Usutu virus.PLoS Negl Trop Dis. 2023 Sep 20;17(9):e0011649. doi: 10.1371/journal.pntd.0011649. eCollection 2023 Sep. PLoS Negl Trop Dis. 2023. PMID: 37729233 Free PMC article.
-
The potential role of the Asian bush mosquito Aedes japonicus as spillover vector for West Nile virus in the Netherlands.Parasit Vectors. 2024 Jun 17;17(1):262. doi: 10.1186/s13071-024-06279-5. Parasit Vectors. 2024. PMID: 38886805 Free PMC article.
-
Competition between two Usutu virus isolates in cell culture and in the common house mosquito Culex pipiens.Front Microbiol. 2023 May 24;14:1195621. doi: 10.3389/fmicb.2023.1195621. eCollection 2023. Front Microbiol. 2023. PMID: 37293213 Free PMC article.
References
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
