Molecular Mechanisms of Skeletal Muscle Hypertrophy
- PMID: 33216041
- PMCID: PMC8075408
- DOI: 10.3233/JND-200568
Molecular Mechanisms of Skeletal Muscle Hypertrophy
Abstract
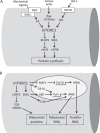
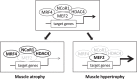
Skeletal muscle hypertrophy can be induced by hormones and growth factors acting directly as positive regulators of muscle growth or indirectly by neutralizing negative regulators, and by mechanical signals mediating the effect of resistance exercise. Muscle growth during hypertrophy is controlled at the translational level, through the stimulation of protein synthesis, and at the transcriptional level, through the activation of ribosomal RNAs and muscle-specific genes. mTORC1 has a central role in the regulation of both protein synthesis and ribosomal biogenesis. Several transcription factors and co-activators, including MEF2, SRF, PGC-1α4, and YAP promote the growth of the myofibers. Satellite cell proliferation and fusion is involved in some but not all muscle hypertrophy models.
Keywords: MEF2; SRF; Skeletal muscle; mTOR; muscle hypertrophy; ribosomal biogenesis; transcriptional control; translational control.
Conflict of interest statement
The authors have no conflict of interest to report.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

