Effectiveness of risk minimisation measures for valproate: A cross-sectional survey among physicians in Europe
- PMID: 33216434
- PMCID: PMC7894483
- DOI: 10.1002/pds.5119
Effectiveness of risk minimisation measures for valproate: A cross-sectional survey among physicians in Europe
Abstract
Purpose: This study evaluated the effectiveness of risk minimisation measures (RMMs) implemented following the 2014 referral for valproate in Europe.
Methods: Cross-sectional survey was conducted over 2-month period in 2016 among physicians who prescribed valproate in France, Germany, the United Kingdom, Spain and Sweden. The web-based questionnaire included five endpoints to evaluate physicians' knowledge on (a) prescribing valproate only for epilepsy and bipolar disorder in women if other treatments were ineffective or not tolerated; (b) ensuring supervision by experienced physicians while treating these conditions; (c) considering alternative treatments for women planning pregnancy, regular review of treatment needs and re-assessing the benefit-risk balance in women and girls reaching puberty; (d) informing patients about the risks of taking valproate during pregnancy and (e) advising women on effective contraception during their treatment.
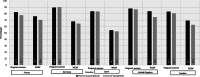
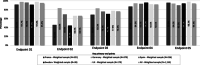
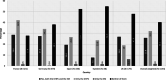
Results: Among 1153 physicians, 95.5% responded prescribing valproate for epilepsy and bipolar disorder in women only if other treatments are ineffective/not tolerated; 66.5% supervised while treatment; 76.6% considered alternative treatments for women planning pregnancy; 92.1% informed patients about the risks of taking valproate during pregnancy and 94.4% advised patients on the use of effective contraception during its treatment. Overall, 25.8% physicians recalled receiving both educational material (EM) and Dear Healthcare Professional Communication (DHPC). All endpoint rates were higher for physicians who acknowledged receipt of both DHPC and EM compared to physicians who did not receive them.
Conclusions: Although results varied across geography and physician speciality, majority of physicians had good knowledge about the indication and safety aspects of prescribing and using valproate.
Keywords: Direct Healthcare Professional Communication; pharmacoepidemiology; prescribing behaviour; risk minimisation measures; valproate.
© 2020 The Authors. Pharmacoepidemiology and Drug Safety published by John Wiley & Sons Ltd.
Conflict of interest statement
M. Toussi, I. Bardoulat are salaried employees of IQVIA (La Défense, France) a human data science company which received funds for the conduct of the study. Stephanie Tcherny‐Lessenot is an employee of Sanofi Aventis R&D (France). Sigalit Kaplan is an employee of Teva Pharmaceutical Industries Ltd. (Israel). Hanka de Voogd is an employee of Mylan EPD (The Netherlands). Vasilis Dimos is an employee of DEMO S.A. (Greece).
Figures


References
-
- European Medicines Agency . Valproate Assessment report 2018. Retrieved on 12 July 2019 from https://www.ema.europa.eu/en/documents/referral/valproate-article-31-ref....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

