The amygdala instructs insular feedback for affective learning
- PMID: 33216712
- PMCID: PMC7679142
- DOI: 10.7554/eLife.60336
The amygdala instructs insular feedback for affective learning
Abstract
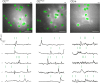
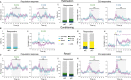
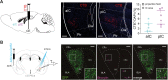
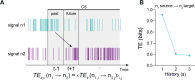
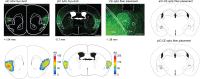
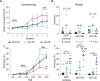
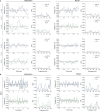
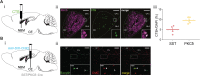
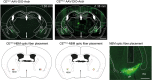
Affective responses depend on assigning value to environmental predictors of threat or reward. Neuroanatomically, this affective value is encoded at both cortical and subcortical levels. However, the purpose of this distributed representation across functional hierarchies remains unclear. Using fMRI in mice, we mapped a discrete cortico-limbic loop between insular cortex (IC), central amygdala (CE), and nucleus basalis of Meynert (NBM), which decomposes the affective value of a conditioned stimulus (CS) into its salience and valence components. In IC, learning integrated unconditioned stimulus (US)-evoked bodily states into CS valence. In turn, CS salience in the CE recruited these CS representations bottom-up via the cholinergic NBM. This way, the CE incorporated interoceptive feedback from IC to improve discrimination of CS valence. Consequently, opto-/chemogenetic uncoupling of hierarchical information flow disrupted affective learning and conditioned responding. Dysfunctional interactions in the IC↔CE/NBM network may underlie intolerance to uncertainty, observed in autism and related psychiatric conditions.
Keywords: affective learning; amygdala; cholinergic basal forebrain; hierarchical interaction; insular cortex; interoceptive value; mouse; neuroscience.
© 2020, Kargl et al.
Conflict of interest statement
DK, JK, SU, FG, LP, JL, KB, WH No competing interests declared
Figures






















References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

