Unique genetic signatures of local adaptation over space and time for diapause, an ecologically relevant complex trait, in Drosophila melanogaster
- PMID: 33216740
- PMCID: PMC7717581
- DOI: 10.1371/journal.pgen.1009110
Unique genetic signatures of local adaptation over space and time for diapause, an ecologically relevant complex trait, in Drosophila melanogaster
Abstract
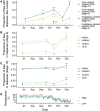
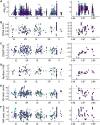
Organisms living in seasonally variable environments utilize cues such as light and temperature to induce plastic responses, enabling them to exploit favorable seasons and avoid unfavorable ones. Local adapation can result in variation in seasonal responses, but the genetic basis and evolutionary history of this variation remains elusive. Many insects, including Drosophila melanogaster, are able to undergo an arrest of reproductive development (diapause) in response to unfavorable conditions. In D. melanogaster, the ability to diapause is more common in high latitude populations, where flies endure harsher winters, and in the spring, reflecting differential survivorship of overwintering populations. Using a novel hybrid swarm-based genome wide association study, we examined the genetic basis and evolutionary history of ovarian diapause. We exposed outbred females to different temperatures and day lengths, characterized ovarian development for over 2800 flies, and reconstructed their complete, phased genomes. We found that diapause, scored at two different developmental cutoffs, has modest heritability, and we identified hundreds of SNPs associated with each of the two phenotypes. Alleles associated with one of the diapause phenotypes tend to be more common at higher latitudes, but these alleles do not show predictable seasonal variation. The collective signal of many small-effect, clinally varying SNPs can plausibly explain latitudinal variation in diapause seen in North America. Alleles associated with diapause are segregating in Zambia, suggesting that variation in diapause relies on ancestral polymorphisms, and both pro- and anti-diapause alleles have experienced selection in North America. Finally, we utilized outdoor mesocosms to track diapause under natural conditions. We found that hybrid swarms reared outdoors evolved increased propensity for diapause in late fall, whereas indoor control populations experienced no such change. Our results indicate that diapause is a complex, quantitative trait with different evolutionary patterns across time and space.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

