Effect of eflornithine on mutation frequency in temozolomide-treated U87MG cells
- PMID: 33216820
- PMCID: PMC7646829
- DOI: 10.18632/oncotarget.27782
Effect of eflornithine on mutation frequency in temozolomide-treated U87MG cells
Abstract
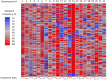
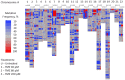
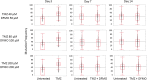
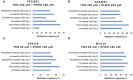
Treatment of infiltrative glioma presents a number of unique challenges due to poor penetration of typical chemotherapeutic agents into the infiltrating edge of tumors. The current chemotherapy options include nitrosoureas (e.g., lomustine) and the imidazotetrazine-class monofunctional DNA alkylating agent, temozolomide (TMZ). Both classes of drugs alkylate DNA and have relatively unrestricted passage from blood into brain where infiltrative tumor cells reside. Recent research indicates that secondary mutations detected in the RB and AKT-mTOR signaling pathways are linked to characteristics of recurrent tumors specific to TMZ-treated patients. It has been hypothesized that a decrease in rate of secondary mutations may result in delay of tumor recurrence. To that end, this study was designed to test viability of decreasing secondary mutations by disrupting the cell division cycle using eflornithine, a specific inhibitor of ornithine decarboxylase. U87MG glioblastoma cell line characterized by chromosomal abnormalities commonly attributed to primary cancers was used as a model for this study. The cells were subjected to TMZ treatment for 3 days followed by eflornithine (DFMO) treatment for 4 or 11 days. It was shown that TMZ significantly increased the frequency of mutations in U87MG glioblastoma cells while DFMO-treated cells showed mutation frequency statistically similar to that of the untreated cells on the respective treatment days. The findings of this study provide evidence to support the hypothesis that DFMO may inhibit progression of DNA mutations caused by alkylating chemotherapy agents, such as TMZ.
Keywords: chemotherapy; eflornithine; glioma; secondary mutations; temozolomide.
Copyright: © 2020 Yam et al.
Conflict of interest statement
CONFLICTS OF INTEREST Authors have no conflicts of interest to declare.
Figures

Similar articles
-
MiR-21 protected human glioblastoma U87MG cells from chemotherapeutic drug temozolomide induced apoptosis by decreasing Bax/Bcl-2 ratio and caspase-3 activity.Brain Res. 2010 Sep 17;1352:255-64. doi: 10.1016/j.brainres.2010.07.009. Epub 2010 Jul 13. Brain Res. 2010. PMID: 20633539
-
Combination treatment for glioblastoma with temozolomide, DFMO and radiation.J BUON. 2019 Jan-Feb;24(1):397-404. J BUON. 2019. PMID: 30941997
-
Phase III randomized study of postradiotherapy chemotherapy with alpha-difluoromethylornithine-procarbazine, N-(2-chloroethyl)-N'-cyclohexyl-N-nitrosurea, vincristine (DFMO-PCV) versus PCV for glioblastoma multiforme.Clin Cancer Res. 2000 Oct;6(10):3878-84. Clin Cancer Res. 2000. PMID: 11051233 Clinical Trial.
-
The Inhibition of microRNA-128 on IGF-1-Activating mTOR Signaling Involves in Temozolomide-Induced Glioma Cell Apoptotic Death.PLoS One. 2016 Nov 28;11(11):e0167096. doi: 10.1371/journal.pone.0167096. eCollection 2016. PLoS One. 2016. PMID: 27893811 Free PMC article.
-
[Treatment of glioma with temozolomide].Brain Nerve. 2009 Jul;61(7):849-54. Brain Nerve. 2009. PMID: 19618863 Review. Japanese.
Cited by
-
Biomolecules to Biomarkers? U87MG Marker Evaluation on the Path towards Glioblastoma Multiforme Pathogenesis.Pharmaceutics. 2024 Jan 18;16(1):123. doi: 10.3390/pharmaceutics16010123. Pharmaceutics. 2024. PMID: 38258133 Free PMC article.
References
-
- Central Brain Tumor Registry of the United States. CBTRUS 2018 Fact Sheet. 2018.
-
- Pletsa V, Valavanis C, van Delft JH, Steenwinkel MJ, Kyrtopoulos SA. DNA damage and mutagenesis induced by procarbazine in lambda lacZ transgenic mice: evidence that bone marrow mutations do not arise primarily through miscoding by O6-methylguanine. Carcinogenesis. 1997; 18:2191–2196. 10.1093/carcin/18.11.2191. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

