Anti-tumor activities of the new oral pan-RAF inhibitor, TAK-580, used as monotherapy or in combination with novel agents in multiple myeloma
- PMID: 33216827
- PMCID: PMC7646837
- DOI: 10.18632/oncotarget.27775
Anti-tumor activities of the new oral pan-RAF inhibitor, TAK-580, used as monotherapy or in combination with novel agents in multiple myeloma
Abstract
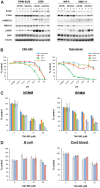
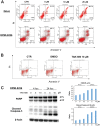
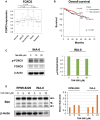
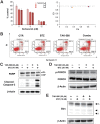
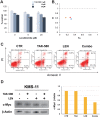
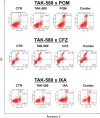
Many RAS pathway inhibitors, including pan-RAF inhibitors, have shown significant anti-tumor activities in both solid and hematological tumors. The pan-RAF inhibitor, TAK-580, is a representative of the novel RAF inhibitors that act by disrupting RAF homo- or heterodimerization. In this study, we examined the anti-tumor effects of TAK-580 used as monotherapy or in combination with bortezomib, lenalidomide, or other novel agents in multiple myeloma (MM) cells in vitro. TAK-580 monotherapy potently targeted proteins in the RAS-RAF-MEK-ERK signaling pathway and induced potent cytotoxicity and apoptosis in MM cell lines and myeloma cells from patients with newly diagnosed and relapsed and/or refractory MM, compared with a representative RAF inhibitor, dabrafenib. Normal donor peripheral blood B lymphocytes and cord blood CD34-positive cells were not affected. Importantly, TAK-580 significantly inhibited phospho-FOXO3 and induced upregulation of BimL and BimS in a dose-dependent manner, finally leading to apoptosis in MM cells. Moreover, TAK-580 enhanced bortezomib-induced cytotoxicity and apoptosis in MM cells via the FOXO3-Bim axis and the terminal unfolded protein response. Importantly, TAK-580 also enhanced lenalidomide-induced cytotoxicity and apoptosis in MM cells. Taken together, our results provide the rationale for TAK-580 monotherapy and/or treatment in combination with novel agents to improve outcomes in patients with MM.
Keywords: Bim; FOXO3a; bortezomib; lenalidomide; pan-RAF inhibitor.
Copyright: © 2020 Suzuki et al.
Conflict of interest statement
CONFLICTS OF INTEREST Authors have no conflicts of interest to declare.
Figures
Similar articles
-
Anti-tumor activity of the pan-RAF inhibitor TAK-580 in combination with KPT-330 (selinexor) in multiple myeloma.Int J Hematol. 2022 Feb;115(2):233-243. doi: 10.1007/s12185-021-03244-1. Epub 2021 Nov 5. Int J Hematol. 2022. PMID: 34741230
-
Combination of a Selective HSP90α/β Inhibitor and a RAS-RAF-MEK-ERK Signaling Pathway Inhibitor Triggers Synergistic Cytotoxicity in Multiple Myeloma Cells.PLoS One. 2015 Dec 2;10(12):e0143847. doi: 10.1371/journal.pone.0143847. eCollection 2015. PLoS One. 2015. PMID: 26630652 Free PMC article.
-
Antitumor activity of the selective pan-RAF inhibitor TAK-632 in BRAF inhibitor-resistant melanoma.Cancer Res. 2013 Dec 1;73(23):7043-55. doi: 10.1158/0008-5472.CAN-13-1825. Epub 2013 Oct 11. Cancer Res. 2013. PMID: 24121489
-
Targeting oncogenic Raf protein-serine/threonine kinases in human cancers.Pharmacol Res. 2018 Sep;135:239-258. doi: 10.1016/j.phrs.2018.08.013. Epub 2018 Aug 15. Pharmacol Res. 2018. PMID: 30118796 Review.
-
Targeting ERK1/2 protein-serine/threonine kinases in human cancers.Pharmacol Res. 2019 Apr;142:151-168. doi: 10.1016/j.phrs.2019.01.039. Epub 2019 Feb 20. Pharmacol Res. 2019. PMID: 30794926 Review.
Cited by
-
Recent Developments in Targeting RAS Downstream Effectors for RAS-Driven Cancer Therapy.Molecules. 2021 Dec 14;26(24):7561. doi: 10.3390/molecules26247561. Molecules. 2021. PMID: 34946644 Free PMC article. Review.
-
A critical review of RAF inhibitors in BRAF-mutated glioma treatment.Pharmacogenomics. 2024;25(7):343-355. doi: 10.1080/14622416.2024.2355859. Epub 2024 Jun 3. Pharmacogenomics. 2024. PMID: 38884947 Free PMC article. Review.
-
Anti-tumor activity of the pan-RAF inhibitor TAK-580 in combination with KPT-330 (selinexor) in multiple myeloma.Int J Hematol. 2022 Feb;115(2):233-243. doi: 10.1007/s12185-021-03244-1. Epub 2021 Nov 5. Int J Hematol. 2022. PMID: 34741230
-
Targeting RAF dimers in RAS mutant tumors: From biology to clinic.Acta Pharm Sin B. 2024 May;14(5):1895-1923. doi: 10.1016/j.apsb.2024.02.018. Epub 2024 Feb 28. Acta Pharm Sin B. 2024. PMID: 38799634 Free PMC article. Review.
-
KRAS and RAS-MAPK Pathway Deregulation in Mature B Cell Lymphoproliferative Disorders.Cancers (Basel). 2022 Jan 28;14(3):666. doi: 10.3390/cancers14030666. Cancers (Basel). 2022. PMID: 35158933 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

