Evaluation of clinical and genetic factors in the population pharmacokinetics of carbamazepine
- PMID: 33217013
- PMCID: PMC8247401
- DOI: 10.1111/bcp.14667
Evaluation of clinical and genetic factors in the population pharmacokinetics of carbamazepine
Abstract
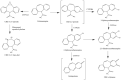
Aims: Carbamazepine can cause hypersensitivity reactions in ~10% of patients. An immunogenic effect can be produced by the electrophilic 10,11-epoxide metabolite but not by carbamazepine. Hypothetically, certain single nucleotide polymorphisms might increase the formation of immunogenic metabolites, leading ultimately to hypersensitivity reactions. This study explores the role of clinical and genetic factors in the pharmacokinetics (PK) of carbamazepine and 3 metabolites known to be chemically reactive or formed through reactive intermediates.
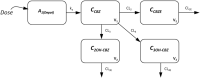
Methods: A combination of rich and sparse PK samples were collected from healthy volunteers and epilepsy patients. All subjects were genotyped for 20 single nucleotide polymorphisms in 11 genes known to be involved in the metabolism or transport of carbamazepine and carbamazepine 10,11-epoxide. Nonlinear mixed effects modelling was used to build a population-PK model.
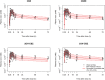
Results: In total, 248 observations were collected from 80 subjects. A 1-compartment PK model with first-order absorption and elimination best described the parent carbamazepine data, with a total clearance of 1.96 L/h, central distribution volume of 164 L and absorption rate constant of 0.45 h-1 . Total daily dose and coadministration of phenytoin were significant covariates for total clearance of carbamazepine. EPHX1-416G/G genotype was a significant covariate for the clearance of carbamazepine 10,11-epoxide.
Conclusion: Our data indicate that carbamazepine clearance was affected by total dose and phenytoin coadministration, but not by genetic factors, while carbamazepine 10,11-epoxide clearance was affected by a variant in the microsomal epoxide hydrolase gene. A much larger sample size would be required to fully evaluate the role of genetic variation in carbamazepine pharmacokinetics, and thereby predisposition to carbamazepine hypersensitivity.
Keywords: carbamazepine; population pharmacokinetics; single nucleotide polymorphisms.
© 2020 The Authors. British Journal of Clinical Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
M.P. receives research funding from various organisations including the MRC, NIHR, EU Commission and Health Education England. He has also received partnership funding for the following: MRC Clinical Pharmacology Training Scheme (cofunded by MRC and Roche, UCB, Eli Lilly and Novartis); a PhD studentship jointly funded by EPSRC and Astra Zeneca; and grant funding from VistaGen Therapeutics. He has also unrestricted educational grant support for the UK Pharmacogenetics and Stratified Medicine Network from Bristol‐Myers Squibb and UCB. He has developed an HLA genotyping panel with MC Diagnostics, but does not benefit financially from this. None of the funding declared above has been used for the current paper.
Figures

References
-
- Pearce RE, Uetrecht JP, Leeder JS. Pathways of carbamazepine bioactivation in vitro: ii. The role of human cytochrome P450 enzymes in the formation of 2‐hydroxyiminostilbene. Drug Metab Dispos. 2005;33(12):1819‐1826. - PubMed
-
- Pearce RE, Vakkalagadda GR, Leeder JS. Pathways of carbamazepine bioactivation in vitro I. characterization of human cytochromes P450 responsible for the formation of 2‐ and 3‐Hydroxylated metabolites. Drug Metab Dispos. 2002;30(11):1170‐1179. - PubMed
-
- Yip VLM, Meng X, Maggs JL, et al. Mass spectrometric characterization of circulating covalent protein adducts derived from epoxide metabolites of carbamazepine in patients. Chem Res Toxicol. 2017;30(7):1419‐1435. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

