Pathogenic Variants in the Myosin Chaperone UNC-45B Cause Progressive Myopathy with Eccentric Cores
- PMID: 33217308
- PMCID: PMC7820787
- DOI: 10.1016/j.ajhg.2020.11.002
Pathogenic Variants in the Myosin Chaperone UNC-45B Cause Progressive Myopathy with Eccentric Cores
Abstract
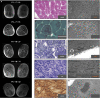
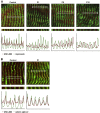
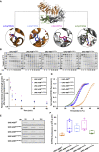
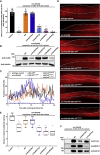
The myosin-directed chaperone UNC-45B is essential for sarcomeric organization and muscle function from Caenorhabditis elegans to humans. The pathological impact of UNC-45B in muscle disease remained elusive. We report ten individuals with bi-allelic variants in UNC45B who exhibit childhood-onset progressive muscle weakness. We identified a common UNC45B variant that acts as a complex hypomorph splice variant. Purified UNC-45B mutants showed changes in folding and solubility. In situ localization studies further demonstrated reduced expression of mutant UNC-45B in muscle combined with abnormal localization away from the A-band towards the Z-disk of the sarcomere. The physiological relevance of these observations was investigated in C. elegans by transgenic expression of conserved UNC-45 missense variants, which showed impaired myosin binding for one and defective muscle function for three. Together, our results demonstrate that UNC-45B impairment manifests as a chaperonopathy with progressive muscle pathology, which discovers the previously unknown conserved role of UNC-45B in myofibrillar organization.
Keywords: C. elegans; UNC-45; UNC45B; chaperone; core myopathy; muscle; myofibrillar; myosin; sarcomere.
Copyright © 2020. Published by Elsevier Inc.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Kim J., Löwe T., Hoppe T. Protein quality control gets muscle into shape. Trends Cell Biol. 2008;18:264–272. - PubMed
-
- Vicart P., Caron A., Guicheney P., Li Z., Prévost M.C., Faure A., Chateau D., Chapon F., Tomé F., Dupret J.M. A missense mutation in the alphaB-crystallin chaperone gene causes a desmin-related myopathy. Nat. Genet. 1998;20:92–95. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

