Trends of microneedle technology in the scientific literature, patents, clinical trials and internet activity
- PMID: 33217629
- PMCID: PMC8042615
- DOI: 10.1016/j.biomaterials.2020.120491
Trends of microneedle technology in the scientific literature, patents, clinical trials and internet activity
Abstract
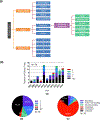
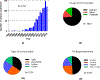
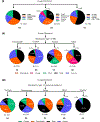
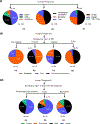
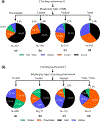
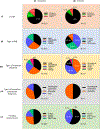
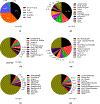
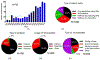
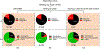
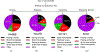
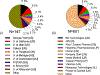
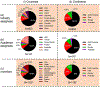
The powerful and intriguing idea that drives the emerging technology of microneedles-shrinking the standard needle to a micron scale-has fostered an entire field of microneedle study and subsequent exponential growth in research and product development. Originally enabled by microfabrication tools derived from the microelectronic industry, microneedles are now produced through a number of methods in a variety of forms including solid, coated, dissolvable, and hollow microneedles. They are used to deliver a broad spectrum of molecules, including small molecules, biomolecules, and vaccines, as well as various forms of energy into the skin, eye, and other tissues. Microneedles are also being exploited for use in diagnostics, as well as additional medical, cosmetic, and other applications. This review elucidates the relative roles of different aspects of microneedle technology development, as shown through scientific papers, patents, clinical studies, and internet/social media activity. Considering >1000 papers, 750 patents, and almost 80 clinical trials, we analyze different attributes of microneedles such as usage of microneedles, types of microneedles, testing environment, types of patent claims, and phases of clinical trials, as well as which institutions and people in academia and industry from different locations and in different journals are publishing, patenting, and otherwise studying the potential of microneedles. We conclude that there is robust and growing activity in the field of microneedles; the technology is rapidly developing and being used for novel applications to benefit human health and well-being.
Keywords: Clinical trial; Diagnostic device; Microneedle; Patent; Skin patch; Transdermal drug delivery.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
7. Competing interest
M.R.P. serves as a consultant to companies, is a founding shareholder of companies, and is an inventor on patents licensed to companies developing microneedle-based products (Micron Biomedical, Clearside Biomedical). These potential conflicts of interest have been disclosed and are managed by Georgia Tech. H.S.G. is a co-inventor on a patent related to coated microneedles and has stock ownership in a startup company called Moonlight Therapeutics that is developing microneedles for food allergy immunotherapy. This conflict of interest has been disclosed and is being managed by Texas Tech University. No financial support was provided by Moonlight Therapeutics for this work.
⊠ The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Figures




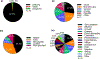
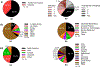
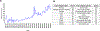
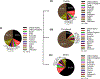

References
-
- Ma G, Wu C, Microneedle, bio-microneedle and bio-inspired microneedle: A review, Journal of Controlled Release 251 (2017) 11–23. - PubMed
-
- Prausnitz MR, Engineering Microneedle Patches for Vaccination and Drug Delivery to Skin, Annual review of chemical and biomolecular engineering 8(1) (2017) 177–200. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

