Molecular Tools for Detection and Identification of Paracoccidioides Species: Current Status and Future Perspectives
- PMID: 33217898
- PMCID: PMC7711936
- DOI: 10.3390/jof6040293
Molecular Tools for Detection and Identification of Paracoccidioides Species: Current Status and Future Perspectives
Abstract
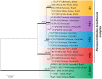
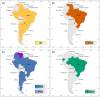
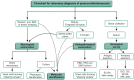
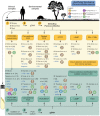
Paracoccidioidomycosis (PCM) is a mycotic disease caused by the Paracoccidioides species, a group of thermally dimorphic fungi that grow in mycelial form at 25 °C and as budding yeasts when cultured at 37 °C or when parasitizing the host tissues. PCM occurs in a large area of Latin America, and the most critical regions of endemicity are in Brazil, Colombia, and Venezuela. The clinical diagnosis of PCM needs to be confirmed through laboratory tests. Although classical laboratory techniques provide valuable information due to the presence of pathognomonic forms of Paracoccidioides spp., nucleic acid-based diagnostics gradually are replacing or complementing culture-based, biochemical, and immunological assays in routine microbiology laboratory practice. Recently, taxonomic changes driven by whole-genomic sequencing of Paracoccidioides have highlighted the need to recognize species boundaries, which could better ascertain Paracoccidioides taxonomy. In this scenario, classical laboratory techniques do not have significant discriminatory power over cryptic agents. On the other hand, several PCR-based methods can detect polymorphisms in Paracoccidioides DNA and thus support species identification. This review is focused on the recent achievements in molecular diagnostics of paracoccidioidomycosis, including the main advantages and pitfalls related to each technique. We discuss these breakthroughs in light of taxonomic changes in the Paracoccidioides genus.
Keywords: Paracoccidioides brasiliensis; Paracoccidioides lutzii; diagnosis; endemic mycosis; epidemiology; molecular diagnostics; paracoccidioidomycosis; systemic mycosis.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
-
- Shikanai-Yasuda M.A., Mendes R.P., Colombo A.L., Queiroz-Telles F., Kono A.S.G., Paniago A.M.M., Nathan A., Valle A., Bagagli E., Benard G., et al. Brazilian guidelines for the clinical management of paracoccidioidomycosis. Rev. Soc. Bras. Med. Trop. 2017;50:715–740. doi: 10.1590/0037-8682-0230-2017. - DOI - PubMed
-
- Do Amaral C.C., Fernandes G.F., Rodrigues A.M., Burger E., de Camargo Z.P. Proteomic analysis of Paracoccidioides brasiliensis complex isolates: Correlation of the levels of differentially expressed proteins with in vivo virulence. PLoS ONE. 2019;14:e0218013. doi: 10.1371/journal.pone.0218013. - DOI - PMC - PubMed
-
- Ambrósio A., Camelo C., Barbosa C., Tomazatti F., Brazões F., Veloso J., Rodrigues G., Rodrigues L., Oliveira P., Aguiar R., et al. Paracoccidioidomycosis disease (Lutz-Splendore-Almeida disease): Additional workup, differential diagnosis, cure control. RMMG-Rev. Méd Minas Gerais. 2014;24:81–92. doi: 10.5935/2238-3182.20140021. - DOI
-
- Restrepo A., Gómez B.L., Tobón A. Paracoccidioidomycosis: Latin America’s own fungal disorder. Curr. Fungal Infect. Rep. 2012;6:303–311. doi: 10.1007/s12281-012-0114-x. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

