Performance of Population Pharmacokinetic Models in Predicting Polymyxin B Exposures
- PMID: 33217914
- PMCID: PMC7698783
- DOI: 10.3390/microorganisms8111814
Performance of Population Pharmacokinetic Models in Predicting Polymyxin B Exposures
Abstract
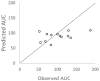
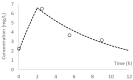
Polymyxin B is the last line of defense in treating multidrug-resistant gram-negative bacterial infections. Dosing of polymyxin B is currently based on total body weight, and a substantial intersubject variability has been reported. We evaluated the performance of different population pharmacokinetic models to predict polymyxin B exposures observed in individual patients. In a prospective observational study, standard dosing (mean 2.5 mg/kg daily) was administered in 13 adult patients. Serial blood samples were obtained at steady state, and plasma polymyxin B concentrations were determined by a validated liquid chromatography tandem mass spectrometry (LC-MS/MS) method. The best-fit estimates of clearance and daily doses were used to derive the observed area under the curve (AUC) in concentration-time profiles. For comparison, 5 different population pharmacokinetic models of polymyxin B were conditioned using patient-specific dosing and demographic (if applicable) variables to predict polymyxin B AUC of the same patient. The predictive performance of the models was assessed by the coefficient of correlation, bias, and precision. The correlations between observed and predicted AUC in all 5 models examined were poor (r2 < 0.2). Nonetheless, the models were reasonable in capturing AUC variability in the patient population. Therapeutic drug monitoring currently remains the only viable approach to individualized dosing.
Keywords: area under the curve; pharmacokinetics; polymyxins; therapeutic drug monitoring.
Conflict of interest statement
The authors declare no conflict of interest. The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
References
-
- Neuner E.A., Yeh J.Y., Hall G.S., Sekeres J., Endimiani A., Bonomo R.A., Shrestha N.K., Fraser T.G., van Duin D. Treatment and outcomes in carbapenem-resistant Klebsiella pneumoniae bloodstream infections. Diagn. Microbiol. Infect. Dis. 2011;69:357–362. doi: 10.1016/j.diagmicrobio.2010.10.013. - DOI - PMC - PubMed
-
- Tsuji B.T., Pogue J.M., Zavascki A.P., Paul M., Daikos G.L., Forrest A., Giacobbe D.R., Viscoli C., Giamarellou H., Karaiskos I., et al. International consensus guidelines for the optimal use of the polymyxins: Endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP) Pharmacotherapy. 2019;39:10–39. - PMC - PubMed
-
- Nelson B.C., Eiras D.P., Gomez-Simmonds A., Loo A.S., Satlin M.J., Jenkins S.G., Whittier S., Calfee D.P., Furuya E.Y., Kubin C.J. Clinical outcomes associated with polymyxin B dose in patients with bloodstream infections due to carbapenem-resistant Gram-negative rods. Antimicrob. Agents Chemother. 2015;59:7000–7006. doi: 10.1128/AAC.00844-15. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

