The Potential Role of Nutraceuticals as an Adjuvant in Breast Cancer Patients to Prevent Hair Loss Induced by Endocrine Therapy
- PMID: 33217935
- PMCID: PMC7698784
- DOI: 10.3390/nu12113537
The Potential Role of Nutraceuticals as an Adjuvant in Breast Cancer Patients to Prevent Hair Loss Induced by Endocrine Therapy
Abstract
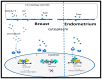
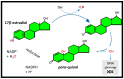
Nutraceuticals, natural dietary and botanical supplements offering health benefits, provide a basis for complementary and alternative medicine (CAM). Use of CAM by healthy individuals and patients with medical conditions is rapidly increasing. For the majority of breast cancer patients, treatment plans involve 5-10 yrs of endocrine therapy, but hair loss/thinning is a common side effect. Many women consider this significant, severely impacting on quality of life, even leading to non-compliance of therapy. Therefore, nutraceuticals that stimulate/maintain hair growth can be proposed. Although nutraceuticals are often available without prescription and taken at the discretion of patients, physicians can be reluctant to recommend them, even as adjuvants, since potential interactions with endocrine therapy have not been fully elucidated. It is, therefore, important to understand the modus operandi of ingredients to be confident that their use will not interfere/interact with therapy. The aim is to improve clinical/healthcare outcomes by combining specific nutraceuticals with conventional care whilst avoiding detrimental interactions. This review presents the current understanding of nutraceuticals beneficial to hair wellness and outcomes concerning efficacy/safety in breast cancer patients. We will focus on describing endocrine therapy and the role of estrogens in cancer and hair growth before evaluating the effects of natural ingredients on breast cancer and hair growth.
Keywords: aromatase inhibitors; breast cancer; endocrine therapy-induced hair loss; estrogen receptor; hair follicle; nutraceuticals; plant extract; tamoxifen.
Conflict of interest statement
G.D. is a consultant at Nutraceuticals Wellness, Inc. and A.R. is an employee at Nutraceuticals Wellness, Inc.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

