Mutant Kras as a Biomarker Plays a Favorable Role in FL118-Induced Apoptosis, Reactive Oxygen Species (ROS) Production and Modulation of Survivin, Mcl-1 and XIAP in Human Bladder Cancer
- PMID: 33217967
- PMCID: PMC7698790
- DOI: 10.3390/cancers12113413
Mutant Kras as a Biomarker Plays a Favorable Role in FL118-Induced Apoptosis, Reactive Oxygen Species (ROS) Production and Modulation of Survivin, Mcl-1 and XIAP in Human Bladder Cancer
Abstract
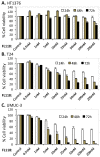
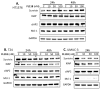
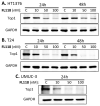
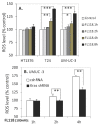
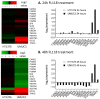
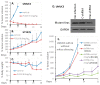
Tumor heterogeneity in key gene mutations in bladder cancer (BC) is a major hurdle for the development of effective treatments. Using molecular, cellular, proteomics and animal models, we demonstrated that FL118, an innovative small molecule, is highly effective at killing T24 and UMUC3 high-grade BC cells, which have Hras and Kras mutations, respectively. In contrast, HT1376 BC cells with wild-type Ras are insensitive to FL118. This concept was further demonstrated in additional BC and colorectal cancer cells with mutant Kras versus those with wild-type Kras. FL118 strongly induced PARP cleavage (apoptosis hallmark) and inhibited survivin, XIAP and/or Mcl-1 in both T24 and UMUC3 cells, but not in the HT1376 cells. Silencing mutant Kras reduced both FL118-induced PARP cleavage and downregulation of survivin, XIAP and Mcl-1 in UMUC3 cells, suggesting mutant Kras is required for FL118 to exhibit higher anticancer efficacy. FL118 increased reactive oxygen species (ROS) production in T24 and UMUC3 cells, but not in HT1376 cells. Silencing mutant Kras in UMUC3 cells reduced FL118-mediated ROS generation. Proteomics analysis revealed that a profound and opposing Kras-relevant signaling protein is changed in UMUC3 cells and not in HT1376 cells. Consistently, in vivo studies indicated that UMUC3 tumors are highly sensitive to FL118 treatment, while HT1376 tumors are highly resistant to this agent. Silencing mutant Kras in UMUC3 cell-derived tumors decreases UMUC3 tumor sensitivity to FL118 treatment. Together, our studies revealed that mutant Kras is a favorable biomarker for FL118 targeted treatment.
Keywords: FL118; Mcl-1; XIAP; apoptosis; bladder cancer; human bladder tumor mouse models; mutant Kras; proteomics analysis; reactive oxygen species (ROS); survivin.
Conflict of interest statement
FL118 and FL118 core structure-based analogs will be further developed in Canget BioTekpharma LLC (
Figures



Similar articles
-
Kidney cancer biomarkers and targets for therapeutics: survivin (BIRC5), XIAP, MCL-1, HIF1α, HIF2α, NRF2, MDM2, MDM4, p53, KRAS and AKT in renal cell carcinoma.J Exp Clin Cancer Res. 2021 Aug 12;40(1):254. doi: 10.1186/s13046-021-02026-1. J Exp Clin Cancer Res. 2021. PMID: 34384473 Free PMC article. Review.
-
Kras mutation subtypes distinctly affect colorectal cancer cell sensitivity to FL118, a novel inhibitor of survivin, Mcl-1, XIAP, cIAP2 and MdmX.Am J Transl Res. 2021 Jul 15;13(7):7458-7474. eCollection 2021. Am J Transl Res. 2021. PMID: 34377229 Free PMC article.
-
FL118, acting as a 'molecular glue degrader', binds to dephosphorylates and degrades the oncoprotein DDX5 (p68) to control c-Myc, survivin and mutant Kras against colorectal and pancreatic cancer with high efficacy.Clin Transl Med. 2022 May;12(5):e881. doi: 10.1002/ctm2.881. Clin Transl Med. 2022. PMID: 35604033 Free PMC article.
-
A novel small molecule FL118 that selectively inhibits survivin, Mcl-1, XIAP and cIAP2 in a p53-independent manner, shows superior antitumor activity.PLoS One. 2012;7(9):e45571. doi: 10.1371/journal.pone.0045571. Epub 2012 Sep 19. PLoS One. 2012. PMID: 23029106 Free PMC article.
-
Discovery of survivin inhibitors and beyond: FL118 as a proof of concept.Int Rev Cell Mol Biol. 2013;305:217-52. doi: 10.1016/B978-0-12-407695-2.00005-6. Int Rev Cell Mol Biol. 2013. PMID: 23890383 Review.
Cited by
-
Structure-Activity Relationship of FL118 Platform Position 7 Versus Position 9-Derived Compounds and Their Mechanism of Action and Antitumor Activity.J Med Chem. 2023 Dec 28;66(24):16888-16916. doi: 10.1021/acs.jmedchem.3c01589. Epub 2023 Dec 15. J Med Chem. 2023. PMID: 38100041 Free PMC article.
-
RalGPS2 Interacts with Akt and PDK1 Promoting Tunneling Nanotubes Formation in Bladder Cancer and Kidney Cells Microenvironment.Cancers (Basel). 2021 Dec 16;13(24):6330. doi: 10.3390/cancers13246330. Cancers (Basel). 2021. PMID: 34944949 Free PMC article.
-
Kidney cancer biomarkers and targets for therapeutics: survivin (BIRC5), XIAP, MCL-1, HIF1α, HIF2α, NRF2, MDM2, MDM4, p53, KRAS and AKT in renal cell carcinoma.J Exp Clin Cancer Res. 2021 Aug 12;40(1):254. doi: 10.1186/s13046-021-02026-1. J Exp Clin Cancer Res. 2021. PMID: 34384473 Free PMC article. Review.
-
Growth Hormone Signaling in Bladder Cancer: Transcriptomic Profiling of Patient Samples and In Vitro Evidence of Therapy Resistance via ABC Transporters and EMT Activation.Int J Mol Sci. 2025 Jul 23;26(15):7113. doi: 10.3390/ijms26157113. Int J Mol Sci. 2025. PMID: 40806252 Free PMC article.
-
Multiple functions of the DEAD-box RNA helicase, DDX5 (p68), make DDX5 a superior oncogenic biomarker and target for targeted cancer therapy.Am J Cancer Res. 2021 Oct 15;11(10):5190-5213. eCollection 2021. Am J Cancer Res. 2021. PMID: 34765320 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

