Assessing Effectiveness of Colonic and Gynecological Risk Reducing Surgery in Lynch Syndrome Individuals
- PMID: 33218006
- PMCID: PMC7698735
- DOI: 10.3390/cancers12113419
Assessing Effectiveness of Colonic and Gynecological Risk Reducing Surgery in Lynch Syndrome Individuals
Erratum in
-
Correction: Dueñas et al. Assessing Effectiveness of Colonic and Gynecological Risk Reducing Surgery in Lynch Syndrome Individuals. Cancers 2020, 12, 3419.Cancers (Basel). 2021 Jun 22;13(13):3104. doi: 10.3390/cancers13133104. Cancers (Basel). 2021. PMID: 34206709 Free PMC article.
Abstract
Background: Colorectal (CRC) and endometrial cancer (EC) are the most common types of cancer in Lynch syndrome (LS). Risk reducing surgeries (RRS) might impact cancer incidence and mortality. Our objectives were to evaluate cumulative incidences of CRC, gynecological cancer and all-cause mortality after RRS in LS individuals.
Methods: Retrospective analysis of 976 LS carriers from a single-institution registry. Primary endpoints were cumulative incidence at 75 years of cancer (metachronous CRC in 425 individuals; EC and ovarian cancer (OC) in 531 individuals) and all-cause mortality cumulative incidence, comparing extended (ES) vs. segmental surgery (SS) in the CRC cohort and risk reducing gynecological surgery (RRGS) vs. surveillance in the gynecological cohort.
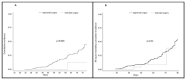
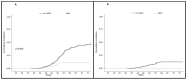
Results: Cumulative incidence at 75 years of metachronous CRC was 12.5% vs. 44.7% (p = 0.04) and all-cause mortality cumulative incidence was 38.6% vs. 55.3% (p = 0.31), for ES and SS, respectively. Cumulative, incidence at 75 years was 11.2% vs. 46.3% for EC (p = 0.001) and 0% vs. 12.7% for OC (p N/A) and all-cause mortality cumulative incidence was 0% vs. 52.7% (p N/A), for RRGS vs. surveillance, respectively.
Conclusions: RRS in LS reduces the incidence of metachronous CRC and gynecological neoplasms, also indicating a reduction in all-cause mortality cumulative incidence in females undergoing RRGS.
Keywords: Lynch syndrome; colorectal neoplasms; endometrial neoplasms; gynecological neoplasms; ovarian neoplasms; prophylactic surgical procedures; risk reducing surgery; risk reduction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Dominguez-Valentin M., Sampson J.R., Seppälä T.T., Broeke S.W.T., Be J.-P.P., Nakken S., Engel C., Aretz S., Jenkins M.A., Sunde L., et al. Correction: Cancer risks by gene, age, and gender in 6350 carriers of pathogenic mismatch repair variants: Findings from the Prospective Lynch Syndrome Database. Genet. Med. 2020;22:15–25. doi: 10.1038/s41436-019-0596-9. - DOI - PMC - PubMed
-
- Møller P., Seppälä T., Bernstein I., Holinski-Feder E., Sala P., Evans D.G., Lindblom A., Macrae F., Blanco I., Sijmons R., et al. Incidence of and survival after subsequent cancers in carriers of pathogenic MMR variants with previous cancer: A report from the prospective Lynch syndrome database. Gut. 2017;66:1657–1664. doi: 10.1136/gutjnl-2016-311403. - DOI - PMC - PubMed
-
- Vasen H., Abdirahman M., Brohet R., Langers A.M.J., Kleibeuker J.H., Van Kouwen M., Koornstra J.J., Boot H., Cats A., Dekker E., et al. One to 2-Year Surveillance Intervals Reduce Risk of Colorectal Cancer in Families with Lynch Syndrome. Gastroenterology. 2010;138:2300–2306. doi: 10.1053/j.gastro.2010.02.053. - DOI - PubMed

