Targeting Adenosine Receptors: A Potential Pharmacological Avenue for Acute and Chronic Pain
- PMID: 33218074
- PMCID: PMC7698931
- DOI: 10.3390/ijms21228710
Targeting Adenosine Receptors: A Potential Pharmacological Avenue for Acute and Chronic Pain
Abstract
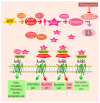
Adenosine is a purine nucleoside, responsible for the regulation of multiple physiological and pathological cellular and tissue functions by activation of four G protein-coupled receptors (GPCR), namely A1, A2A, A2B, and A3 adenosine receptors (ARs). In recent years, extensive progress has been made to elucidate the role of adenosine in pain regulation. Most of the antinociceptive effects of adenosine are dependent upon A1AR activation located at peripheral, spinal, and supraspinal sites. The role of A2AAR and A2BAR is more controversial since their activation has both pro- and anti-nociceptive effects. A3AR agonists are emerging as promising candidates for neuropathic pain. Although their therapeutic potential has been demonstrated in diverse preclinical studies, no AR ligands have so far reached the market. To date, novel pharmacological approaches such as adenosine regulating agents and allosteric modulators have been proposed to improve efficacy and limit side effects enhancing the effect of endogenous adenosine. This review aims to provide an overview of the therapeutic potential of ligands interacting with ARs and the adenosinergic system for the treatment of acute and chronic pain.
Keywords: adenosine; adenosine receptors; antinociception; pain.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

