Exploiting Mannuronan C-5 Epimerases in Commercial Alginate Production
- PMID: 33218095
- PMCID: PMC7698916
- DOI: 10.3390/md18110565
Exploiting Mannuronan C-5 Epimerases in Commercial Alginate Production
Abstract
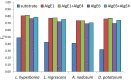
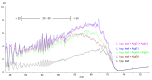
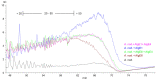
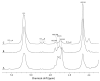
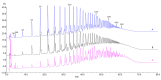
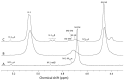
Alginates are one of the major polysaccharide constituents of marine brown algae in commercial manufacturing. However, the content and composition of alginates differ according to the distinct parts of these macroalgae and have a direct impact on the concentration of guluronate and subsequent commercial value of the final product. The Azotobacter vinelandii mannuronan C-5 epimerases AlgE1 and AlgE4 were used to determine their potential value in tailoring the production of high guluronate low-molecular-weight alginates from two sources of high mannuronic acid alginates, the naturally occurring harvested brown algae (Ascophyllum nodosum, Durvillea potatorum, Laminaria hyperborea and Lessonia nigrescens) and a pure mannuronic acid alginate derived from fermented production of the mutant strain of Pseudomonas fluorescens NCIMB 10,525. The mannuronan C-5 epimerases used in this study increased the content of guluronate from 32% up to 81% in both the harvested seaweed and bacterial fermented alginate sources. The guluronate-rich alginate oligomers subsequently derived from these two different sources showed structural identity as determined by proton nuclear magnetic resonance (1H NMR), high-performance anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD) and size-exclusion chromatography with online multi-angle static laser light scattering (SEC-MALS). Functional identity was determined by minimum inhibitory concentration (MIC) assays with selected bacteria and antibiotics using the previously documented low-molecular-weight guluronate enriched alginate OligoG CF-5/20 as a comparator. The alginates produced using either source showed similar antibiotic potentiation effects to the drug candidate OligoG CF-5/20 currently in development as a mucolytic and anti-biofilm agent. These findings clearly illustrate the value of using epimerases to provide an alternative production route for novel low-molecular-weight alginates.
Keywords: guluronate oligomers; mannuronan C-5 epimerases; potentiation of antibiotic effect; seaweed and bacterial alginate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Drummond D.W., Hirst E.L., Percival E. The constitution of alginic acid. J. Chem. Soc. 1962:1208–1216. doi: 10.1039/jr9620001208. - DOI
-
- Haug A., Larsen B., Smidsrød O. Uronic acid sequence in alginate from different sources. Carbohydrate Res. 1974;32:217–225. doi: 10.1016/S0008-6215(00)82100-X. - DOI
-
- Smidsrød O., Draget K.I. Chemistry and physical properties of alginates. Carbohydr. Eur. 1996;14:6–13.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

