The Δ133p53 Isoforms, Tuners of the p53 Pathway
- PMID: 33218139
- PMCID: PMC7698932
- DOI: 10.3390/cancers12113422
The Δ133p53 Isoforms, Tuners of the p53 Pathway
Abstract
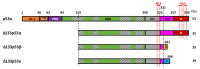
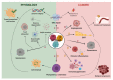
The TP53 gene is a critical tumor suppressor and key determinant of cell fate which regulates numerous cellular functions including DNA repair, cell cycle arrest, cellular senescence, apoptosis, autophagy and metabolism. In the last 15 years, the p53 pathway has grown in complexity through the discovery that TP53 differentially expresses twelve p53 protein isoforms in human cells with both overlapping and unique biologic activities. Here, we summarize the current knowledge on the Δ133p53 isoforms (Δ133p53α, Δ133p53β and Δ133p53γ), which are evolutionary derived and found only in human and higher order primates. All three isoforms lack both of the transactivation domains and the beginning of the DNA-binding domain. Despite the absence of these canonical domains, the Δ133p53 isoforms maintain critical functions in cancer, physiological and premature aging, neurodegenerative diseases, immunity and inflammation, and tissue repair. The ability of the Δ133p53 isoforms to modulate the p53 pathway functions underscores the need to include these p53 isoforms in our understanding of how the p53 pathway contributes to multiple physiological and pathological mechanisms. Critically, further characterization of p53 isoforms may identify novel regulatory modes of p53 pathway functions that contribute to disease progression and facilitate the development of new therapeutic strategies.
Keywords: aging; cancer; p53; p53 isoforms; Δ133p53.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The p53 isoform, Δ133p53α, stimulates angiogenesis and tumour progression.Oncogene. 2013 Apr 25;32(17):2150-60. doi: 10.1038/onc.2012.242. Epub 2012 Jun 25. Oncogene. 2013. PMID: 22733133
-
p53 regulates the transcription of its Delta133p53 isoform through specific response elements contained within the TP53 P2 internal promoter.Oncogene. 2010 May 6;29(18):2691-700. doi: 10.1038/onc.2010.26. Epub 2010 Mar 1. Oncogene. 2010. PMID: 20190805
-
Δ133p53 represses p53-inducible senescence genes and enhances the generation of human induced pluripotent stem cells.Cell Death Differ. 2017 Jun;24(6):1017-1028. doi: 10.1038/cdd.2017.48. Epub 2017 Mar 31. Cell Death Differ. 2017. PMID: 28362428 Free PMC article.
-
p53 Isoforms in Cellular Senescence- and Ageing-Associated Biological and Physiological Functions.Int J Mol Sci. 2019 Nov 29;20(23):6023. doi: 10.3390/ijms20236023. Int J Mol Sci. 2019. PMID: 31795382 Free PMC article. Review.
-
p53 isoforms gain functions.Oncogene. 2010 Sep 16;29(37):5113-9. doi: 10.1038/onc.2010.266. Epub 2010 Jul 12. Oncogene. 2010. PMID: 20622898 Review.
Cited by
-
Banp regulates DNA damage response and chromosome segregation during the cell cycle in zebrafish retina.Elife. 2022 Aug 9;11:e74611. doi: 10.7554/eLife.74611. Elife. 2022. PMID: 35942692 Free PMC article.
-
Δ133p53α Protects Human Astrocytes from Amyloid-beta Induced Senescence and Neurotoxicity.Neuroscience. 2022 Aug 21;498:190-202. doi: 10.1016/j.neuroscience.2022.06.004. Epub 2022 Jun 15. Neuroscience. 2022. PMID: 35716965 Free PMC article.
-
Δ133p53 coordinates ECM-driven morphogenesis and gene expression in three-dimensional mammary epithelial acini.J Cell Sci. 2022 Nov 1;135(21):jcs259673. doi: 10.1242/jcs.259673. Epub 2022 Nov 4. J Cell Sci. 2022. PMID: 36239052 Free PMC article.
-
p53 and Its Isoforms in Renal Cell Carcinoma-Do They Matter?Biomedicines. 2022 Jun 6;10(6):1330. doi: 10.3390/biomedicines10061330. Biomedicines. 2022. PMID: 35740352 Free PMC article. Review.
-
It is not all about the alpha: elevated expression of p53β variants is associated with lower probability of survival in a retrospective melanoma cohort.Cancer Cell Int. 2023 Oct 4;23(1):228. doi: 10.1186/s12935-023-03083-6. Cancer Cell Int. 2023. PMID: 37794430 Free PMC article.
References
-
- Lozano G., Levine A.J. The p53 Protein: From Cell Regulation to Cancer. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY, USA: 2016. A Cold Spring Harbor Perspectives in Medicine Collection.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

