Analysis of Measles-Mumps-Rubella (MMR) Titers of Recovered COVID-19 Patients
- PMID: 33219096
- PMCID: PMC7686805
- DOI: 10.1128/mBio.02628-20
Analysis of Measles-Mumps-Rubella (MMR) Titers of Recovered COVID-19 Patients
Abstract
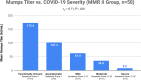
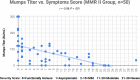
The measles-mumps-rubella (MMR) vaccine has been theorized to provide protection against coronavirus disease 2019 (COVID-19). Our aim was to determine whether any MMR IgG titers are inversely correlated with severity in recovered COVID-19 patients previously vaccinated with MMR II. We divided 80 subjects into two groups, comparing MMR titers to recent COVID-19 severity levels. The MMR II group consisted of 50 subjects who would primarily have MMR antibodies from the MMR II vaccine, and a comparison group of 30 subjects consisted of those who would primarily have MMR antibodies from sources other than MMR II, including prior measles, mumps, and/or rubella illnesses. There was a significant inverse correlation (rs = -0.71, P < 0.001) between mumps virus titers (mumps titers) and COVID-19 severity within the MMR II group. There were no significant correlations between mumps titers and severity in the comparison group, between mumps titers and age in the MMR II group, or between severity and measles or rubella titers in either group. Within the MMR II group, mumps titers of 134 to 300 arbitrary units (AU)/ml (n = 8) were found only in those who were functionally immune or asymptomatic; all with mild symptoms had mumps titers below 134 AU/ml (n = 17); all with moderate symptoms had mumps titers below 75 AU/ml (n = 11); all who had been hospitalized and had required oxygen had mumps titers below 32 AU/ml (n = 5). Our results demonstrate that there is a significant inverse correlation between mumps titers from MMR II and COVID-19 severity.IMPORTANCE COVID-19 has presented various paradoxes that, if understood better, may provide clues to controlling the pandemic, even before a COVID-19 vaccine is widely available. First, young children are largely spared from severe disease. Second, numerous countries have COVID-19 death rates that are as low as 1% of the death rates of other countries. Third, many people, despite prolonged close contact with someone who is COVID-19 positive, never test positive themselves. Fourth, nearly half of people who test positive for COVID-19 are asymptomatic. Some researchers have theorized that the measles-mumps-rubella (MMR) vaccine may be responsible for these disparities. The significance of our study is that it showed that mumps titers related to the MMR II vaccine are significantly and inversely correlated with the severity of COVID-19-related symptoms, supporting the theorized association between the MMR vaccine and COVID-19 severity.
Keywords: COVID-19; MMR; SARS-CoV-2; coronavirus; immunization; measles; mumps; rubella; titers; vaccines.
Copyright © 2020 Gold et al.
Figures
Comment in
-
MMR Vaccine and COVID-19: Measles Protein Homology May Contribute to Cross-Reactivity or to Complement Activation Protection.mBio. 2021 Feb 2;12(1):e03447-20. doi: 10.1128/mBio.03447-20. mBio. 2021. PMID: 33531391 Free PMC article. No abstract available.
-
Reply to Marakasova and Baranova, "MMR Vaccine and COVID-19: Measles Protein Homology May Contribute to Cross-Reactivity or to Complement Activation Protection".mBio. 2021 Feb 2;12(1):e03682-20. doi: 10.1128/mBio.03682-20. mBio. 2021. PMID: 33531392 Free PMC article. No abstract available.
Similar articles
-
Measles-mumps-rubella booster and post-COVID-19 immunity: A retrospective cohort study.Vaccine. 2025 May 31;57:127232. doi: 10.1016/j.vaccine.2025.127232. Epub 2025 May 12. Vaccine. 2025. PMID: 40359811
-
Detection of serum antibodies against measles, mumps and rubella after primary measles, mumps and rubella (MMR) vaccination in children.Arch Iran Med. 2013 Jan;16(1):38-41. Arch Iran Med. 2013. PMID: 23273235
-
Assessment of immune status against measles, mumps, and rubella in young Kuwaitis: MMR vaccine efficacy.J Med Virol. 2020 Aug;92(8):963-970. doi: 10.1002/jmv.25665. Epub 2020 Feb 3. J Med Virol. 2020. PMID: 31919861
-
Immunogenicity and persistence of trivalent measles, mumps, and rubella vaccines: a systematic review and meta-analysis.Lancet Infect Dis. 2021 Feb;21(2):286-295. doi: 10.1016/S1473-3099(20)30442-4. Epub 2020 Sep 1. Lancet Infect Dis. 2021. PMID: 32888410 Free PMC article.
-
Measles, mumps, rubella vaccine (Priorix; GSK-MMR): a review of its use in the prevention of measles, mumps and rubella.Drugs. 2003;63(19):2107-26. doi: 10.2165/00003495-200363190-00012. Drugs. 2003. PMID: 12962524 Review.
Cited by
-
Human health implications of emerging diseases and the current situation in India's vaccine industry.Sci One Health. 2023 Oct 29;2:100046. doi: 10.1016/j.soh.2023.100046. eCollection 2023. Sci One Health. 2023. PMID: 39077045 Free PMC article. Review.
-
Does Measles, Mumps, and Rubella (MMR) Vaccination Protect against COVID-19 Outcomes: A Nationwide Cohort Study.Vaccines (Basel). 2022 Nov 16;10(11):1938. doi: 10.3390/vaccines10111938. Vaccines (Basel). 2022. PMID: 36423033 Free PMC article.
-
Identification of Risk Factors for COVID-19 Hospitalization in Patients With Anti-Rheumatic Drugs: Results From a Multicenter Nested Case Control Study.Clin Pharmacol Ther. 2022 May;111(5):1061-1065. doi: 10.1002/cpt.2551. Epub 2022 Feb 23. Clin Pharmacol Ther. 2022. PMID: 35143039 Free PMC article.
-
Measles, mumps and rubella vaccine and heterologous immunity: a way out of the COVID-19 crisis?Sudan J Paediatr. 2022;22(1):10-18. doi: 10.24911/SJP.106-1621869672. Sudan J Paediatr. 2022. PMID: 35958081 Free PMC article. Review.
-
Multidimensional futuristic approaches to address the pandemics beyond COVID-19.Heliyon. 2023 Jun;9(6):e17148. doi: 10.1016/j.heliyon.2023.e17148. Epub 2023 Jun 11. Heliyon. 2023. PMID: 37325452 Free PMC article. Review.
References
-
- Pawlowski C, Puranik A, Bandi H, Venkatakrishnan AJ, Agarwal V, Kennedy R, O’Horo JC, Gorres GJ, Williams AW, Halamka J, Badley AD, Soundararajan V. 2020. Exploratory analysis of immunization records highlights decreased SARS-CoV-2 rates in individuals with recent non-COVID-19 vaccinations. MedRxiv doi:10.1101/2020.07.27.20161976. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

