VZHE-039, a novel antisickling agent that prevents erythrocyte sickling under both hypoxic and anoxic conditions
- PMID: 33219275
- PMCID: PMC7679387
- DOI: 10.1038/s41598-020-77171-2
VZHE-039, a novel antisickling agent that prevents erythrocyte sickling under both hypoxic and anoxic conditions
Abstract
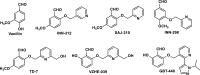
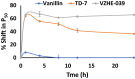
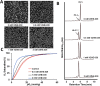
Sickle cell disease (SCD) results from a hemoglobin (Hb) mutation βGlu6 → βVal6 that changes normal Hb (HbA) into sickle Hb (HbS). Under hypoxia, HbS polymerizes into rigid fibers, causing red blood cells (RBCs) to sickle; leading to numerous adverse pathological effects. The RBC sickling is made worse by the low oxygen (O2) affinity of HbS, due to elevated intra-RBC concentrations of the natural Hb effector, 2,3-diphosphoglycerate. This has prompted the development of Hb modifiers, such as aromatic aldehydes, with the intent of increasing Hb affinity for O2 with subsequent prevention of RBC sickling. One such molecule, Voxelotor was recently approved by U.S. FDA to treat SCD. Here we report results of a novel aromatic aldehyde, VZHE-039, that mimics both the O2-dependent and O2-independent antisickling properties of fetal hemoglobin. The latter mechanism of action-as elucidated through crystallographic and biological studies-is likely due to disruption of key intermolecular contacts necessary for stable HbS polymer formation. This dual antisickling mechanism, in addition to VZHE-039 metabolic stability, has translated into significantly enhanced and sustained pharmacologic activities. Finally, VZHE-039 showed no significant inhibition of several CYPs, demonstrated efficient RBC partitioning and high membrane permeability, and is not an efflux transporter (P-gp) substrate.
Conflict of interest statement
The authors declare no competing interests. Virginia Commonwealth University has filed a patent related to TD-7 and VZHE-039 and licensed to Illexcor Therapeutics.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

