Diagnostic Accuracy of Amyloid versus 18 F-Fluorodeoxyglucose Positron Emission Tomography in Autopsy-Confirmed Dementia
- PMID: 33219525
- PMCID: PMC7856004
- DOI: 10.1002/ana.25968
Diagnostic Accuracy of Amyloid versus 18 F-Fluorodeoxyglucose Positron Emission Tomography in Autopsy-Confirmed Dementia
Abstract
Objective: The purpose of this study was to compare the diagnostic accuracy of antemortem 11 C-Pittsburgh compound B (PIB) and 18 F-fluorodeoxyglucose (FDG) positron emission tomography (PET) versus autopsy diagnosis in a heterogenous sample of patients.
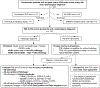
Methods: One hundred one participants underwent PIB and FDG PET during life and neuropathological assessment. PET scans were visually interpreted by 3 raters blinded to clinical information. PIB PET was rated as positive or negative for cortical retention, whereas FDG scans were read as showing an Alzheimer disease (AD) or non-AD pattern. Neuropathological diagnoses were assigned using research criteria. Majority visual reads were compared to intermediate-high AD neuropathological change (ADNC).
Results: One hundred one participants were included (mean age = 67.2 years, 41 females, Mini-Mental State Examination = 21.9, PET-to-autopsy interval = 4.4 years). At autopsy, 32 patients showed primary AD, 56 showed non-AD neuropathology (primarily frontotemporal lobar degeneration [FTLD]), and 13 showed mixed AD/FTLD pathology. PIB showed higher sensitivity than FDG for detecting intermediate-high ADNC (96%, 95% confidence interval [CI] = 89-100% vs 80%, 95% CI = 68-92%, p = 0.02), but equivalent specificity (86%, 95% CI = 76-95% vs 84%, 95% CI = 74-93%, p = 0.80). In patients with congruent PIB and FDG reads (77/101), combined sensitivity was 97% (95% CI = 92-100%) and specificity was 98% (95% CI = 93-100%). Nine of 24 patients with incongruent reads were found to have co-occurrence of AD and non-AD pathologies.
Interpretation: In our sample enriched for younger onset cognitive impairment, PIB-PET had higher sensitivity than FDG-PET for intermediate-high ADNC, with similar specificity. When both modalities are congruent, sensitivity and specificity approach 100%, whereas mixed pathology should be considered when PIB and FDG are incongruent. ANN NEUROL 2021;89:389-401.
© 2020 American Neurological Association.
Conflict of interest statement
Potential conflict of interests:
Adam Boxer and Lea T Grinberg - Dr. Boxer and Dr. Greenberg receives research support from Avid Radiopharmaceuticals, Eli Lilly, that develop amyloid PET ligand for commercial distribution in clinical care, not used in this study.
Gil D Rabinovici – Dr. Rabinovici receives research support from Avid Radiopharmaceuticals, Eli Lilly, GE Healthcare and Life Molecular Imaging, that develop amyloid PET ligands for commercial distribution in clinical care, not used in this study. He has received speaking honoraria from GE Healthcare. The remaining authors have nothing to report.
Figures


Similar articles
-
Amyloid vs FDG-PET in the differential diagnosis of AD and FTLD.Neurology. 2011 Dec 6;77(23):2034-42. doi: 10.1212/WNL.0b013e31823b9c5e. Epub 2011 Nov 30. Neurology. 2011. PMID: 22131541 Free PMC article.
-
Early 11C-PIB frames and 18F-FDG PET measures are comparable: a study validated in a cohort of AD and FTLD patients.J Nucl Med. 2011 Feb;52(2):173-9. doi: 10.2967/jnumed.110.082057. Epub 2011 Jan 13. J Nucl Med. 2011. PMID: 21233181 Free PMC article.
-
Positron Emission Tomography Imaging With [18F]flortaucipir and Postmortem Assessment of Alzheimer Disease Neuropathologic Changes.JAMA Neurol. 2020 Jul 1;77(7):829-839. doi: 10.1001/jamaneurol.2020.0528. JAMA Neurol. 2020. PMID: 32338734 Free PMC article.
-
Positron emission tomography radiopharmaceuticals for imaging brain Beta-amyloid.Semin Nucl Med. 2011 Jul;41(4):283-99. doi: 10.1053/j.semnuclmed.2011.02.005. Semin Nucl Med. 2011. PMID: 21624562 Review.
-
Diagnostic accuracy of 18 F-FDG and 11 C-PIB-PET for prediction of short-term conversion to Alzheimer's disease in subjects with mild cognitive impairment.Int J Clin Pract. 2012 Feb;66(2):185-98. doi: 10.1111/j.1742-1241.2011.02845.x. Int J Clin Pract. 2012. PMID: 22257044 Review.
Cited by
-
Head-to-head comparison between plasma p-tau217 and flortaucipir-PET in amyloid-positive patients with cognitive impairment.Alzheimers Res Ther. 2023 Sep 22;15(1):157. doi: 10.1186/s13195-023-01302-w. Alzheimers Res Ther. 2023. PMID: 37740209 Free PMC article.
-
Neuroimaging in Frontotemporal Dementia: Heterogeneity and Relationships with Underlying Neuropathology.Neurotherapeutics. 2021 Apr;18(2):728-752. doi: 10.1007/s13311-021-01101-x. Epub 2021 Aug 13. Neurotherapeutics. 2021. PMID: 34389969 Free PMC article. Review.
-
Donanemab: Appropriate use recommendations.J Prev Alzheimers Dis. 2025 May;12(5):100150. doi: 10.1016/j.tjpad.2025.100150. Epub 2025 Mar 27. J Prev Alzheimers Dis. 2025. PMID: 40155270 Free PMC article.
-
The role of sleep in Alzheimer's disease: a mini review.Front Neurosci. 2025 Feb 5;19:1428733. doi: 10.3389/fnins.2025.1428733. eCollection 2025. Front Neurosci. 2025. PMID: 39975973 Free PMC article. Review.
-
Extracellular vesicles as nanotheranostic platforms for targeted neurological disorder interventions.Nano Converg. 2024 May 13;11(1):19. doi: 10.1186/s40580-024-00426-5. Nano Converg. 2024. PMID: 38739358 Free PMC article. Review.
References
-
- Galton CJ, Patterson K, Xuereb JH, Hodges JR. Atypical and typical presentations of Alzheimer’s disease: a clinical, neuropsychological, neuroimaging and pathological study of 13 cases. Brain J Neurol 2000; 123 Pt 3: 484–98. - PubMed
-
- Graham A, Davies R, Xuereb J, et al. Pathologically proven frontotemporal dementia presenting with severe amnesia. Brain J Neurol 2005; 128: 597–605. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 AG023501/AG/NIA NIH HHS/United States
- K24 AG045333/AG/NIA NIH HHS/United States
- K24 AG053435/AG/NIA NIH HHS/United States
- R01 AG038791/AG/NIA NIH HHS/United States
- P30 AG062422/AG/NIA NIH HHS/United States
- P01 AG019724/AG/NIA NIH HHS/United States
- R01 AG032306/AG/NIA NIH HHS/United States
- R01 NS050915/NS/NINDS NIH HHS/United States
- K99 AG065501/AG/NIA NIH HHS/United States
- K08 AG052648/AG/NIA NIH HHS/United States
- P30 AG010129/AG/NIA NIH HHS/United States
- R01 AG055121/AG/NIA NIH HHS/United States
- R01 AG045611/AG/NIA NIH HHS/United States
- K24 DC015544/DC/NIDCD NIH HHS/United States

