Interleukin-33 Promotes Serotonin Release from Enterochromaffin Cells for Intestinal Homeostasis
- PMID: 33220232
- PMCID: PMC7856083
- DOI: 10.1016/j.immuni.2020.10.014
Interleukin-33 Promotes Serotonin Release from Enterochromaffin Cells for Intestinal Homeostasis
Abstract
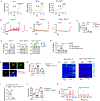
The gastrointestinal tract is known as the largest endocrine organ that encounters and integrates various immune stimulations and neuronal responses due to constant environmental challenges. Enterochromaffin (EC) cells, which function as chemosensors on the gut epithelium, are known to translate environmental cues into serotonin (5-HT) production, contributing to intestinal physiology. However, how immune signals participate in gut sensation and neuroendocrine response remains unclear. Interleukin-33 (IL-33) acts as an alarmin cytokine by alerting the system of potential environmental stresses. We here demonstrate that IL-33 induced instantaneous peristaltic movement and facilitated Trichuris muris expulsion. We found that IL-33 could be sensed by EC cells, inducing release of 5-HT. IL-33-mediated 5-HT release activated enteric neurons, subsequently promoting gut motility. Mechanistically, IL-33 triggered calcium influx via a non-canonical signaling pathway specifically in EC cells to induce 5-HT secretion. Our data establish an immune-neuroendocrine axis in calibrating rapid 5-HT release for intestinal homeostasis.
Keywords: IL-33; PLC-γ1; ST2; TRPA1; enterochromaffin cells; gut motility; helminth clearance; serotonin release.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures
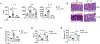





Comment in
-
IL-33 Changes Our "Gut Feelings" about Serotonin.Immunity. 2021 Jan 12;54(1):9-11. doi: 10.1016/j.immuni.2020.12.007. Immunity. 2021. PMID: 33440138
References
-
- Alcaino C, Knutson KR, Treichel AJ, Yildiz G, Strege PR, Linden DR, Li JH, Leiter AB, Szurszewski JH, Farrugia G, and Beyder A (2018). A population of gut epithelial enterochromaffin cells is mechanosensitive and requires Piezo2 to convert force into serotonin release. Proceedings of the National Academy of Sciences of the United States of America 115, E7632–E7641. - PMC - PubMed
-
- Basak O, Beumer J, Wiebrands K, Seno H, van Oudenaarden A, and Clevers H (2017). Induced Quiescence of Lgr5+ Stem Cells in Intestinal Organoids Enables Differentiation of Hormone-Producing Enteroendocrine Cells. Cell Stem Cell 20, 177–190 e174. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

