Increasing SARS-CoV-2 RT-qPCR testing capacity by sample pooling
- PMID: 33220439
- PMCID: PMC7674967
- DOI: 10.1016/j.ijid.2020.11.155
Increasing SARS-CoV-2 RT-qPCR testing capacity by sample pooling
Abstract
Objectives: Limited testing capacity has characterized the ongoing coronavirus disease 2019 (COVID-19) pandemic in Spain, hampering timely control of outbreaks and opportunities to reduce the escalation of community transmission. This study investigated the potential to use sample pooling, followed by one-step retrotranscription and real-time quantitative reverse transcription polymerase chain reaction (RT-qPCR) to increase testing capacity for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2).
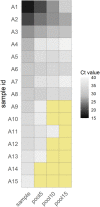
Methods: Various pool sizes (five, 10 and 15 samples) were evaluated prior to RNA extraction followed by standard RT-qPCR for the diagnosis of COVID-19. The pool size achieving reproducible results with individual sample testing was subsequently used to assess nasopharyngeal samples in a tertiary hospital in August 2020.
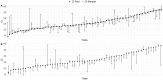
Results: A pool size of five samples had higher sensitivity compared with pool sizes of 10 and 15 samples, showing a mean cycle threshold (Ct) shift of 3.5 [standard deviation (SD) 2.2] between the pooled test and positive samples in the pool. Next, a pool size of five was used to test a total of 895 pools (4475 prospective samples) using two different RT-qPCR kits. The Real Accurate Quadruplex corona-plus PCR Kit (PathoFinder) reported the lowest mean Ct shift [2.2 (SD 2.4)] between the pool and individual samples. This strategy enables detection of individual positive samples in positive pools with Ct of 16.7-39.4.
Conclusions: Grouping samples into pools of five for RT-qPCR resulted in an increase in SARS-CoV-2 testing capacity with minimal loss of sensitivity compared with testing each sample individually.
Keywords: COVID-19; SARS-CoV-2; Sample pooling; Scalability; Testing capacity.
Copyright © 2020 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures
References
-
- FDA COVID-19 Update . 2020. FDA issues first emergency authorization for sample pooling in diagnostic testing.https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19...
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

