Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial
- PMID: 33220855
- PMCID: PMC7674972
- DOI: 10.1016/S0140-6736(20)32466-1
Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial
Erratum in
-
Department of Error.Lancet. 2021 Dec 19;396(10267):1978. doi: 10.1016/S0140-6736(20)32678-7. Lancet. 2021. PMID: 33341142 Free PMC article. No abstract available.
-
Department of Error.Lancet. 2021 Apr 10;397(10282):1350. doi: 10.1016/S0140-6736(21)00777-7. Lancet. 2021. PMID: 33838756 Free PMC article. No abstract available.
Abstract
Background: Older adults (aged ≥70 years) are at increased risk of severe disease and death if they develop COVID-19 and are therefore a priority for immunisation should an efficacious vaccine be developed. Immunogenicity of vaccines is often worse in older adults as a result of immunosenescence. We have reported the immunogenicity of a novel chimpanzee adenovirus-vectored vaccine, ChAdOx1 nCoV-19 (AZD1222), in young adults, and now describe the safety and immunogenicity of this vaccine in a wider range of participants, including adults aged 70 years and older.
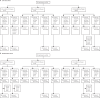
Methods: In this report of the phase 2 component of a single-blind, randomised, controlled, phase 2/3 trial (COV002), healthy adults aged 18 years and older were enrolled at two UK clinical research facilities, in an age-escalation manner, into 18-55 years, 56-69 years, and 70 years and older immunogenicity subgroups. Participants were eligible if they did not have severe or uncontrolled medical comorbidities or a high frailty score (if aged ≥65 years). First, participants were recruited to a low-dose cohort, and within each age group, participants were randomly assigned to receive either intramuscular ChAdOx1 nCoV-19 (2·2 × 1010 virus particles) or a control vaccine, MenACWY, using block randomisation and stratified by age and dose group and study site, using the following ratios: in the 18-55 years group, 1:1 to either two doses of ChAdOx1 nCoV-19 or two doses of MenACWY; in the 56-69 years group, 3:1:3:1 to one dose of ChAdOx1 nCoV-19, one dose of MenACWY, two doses of ChAdOx1 nCoV-19, or two doses of MenACWY; and in the 70 years and older, 5:1:5:1 to one dose of ChAdOx1 nCoV-19, one dose of MenACWY, two doses of ChAdOx1 nCoV-19, or two doses of MenACWY. Prime-booster regimens were given 28 days apart. Participants were then recruited to the standard-dose cohort (3·5-6·5 × 1010 virus particles of ChAdOx1 nCoV-19) and the same randomisation procedures were followed, except the 18-55 years group was assigned in a 5:1 ratio to two doses of ChAdOx1 nCoV-19 or two doses of MenACWY. Participants and investigators, but not staff administering the vaccine, were masked to vaccine allocation. The specific objectives of this report were to assess the safety and humoral and cellular immunogenicity of a single-dose and two-dose schedule in adults older than 55 years. Humoral responses at baseline and after each vaccination until 1 year after the booster were assessed using an in-house standardised ELISA, a multiplex immunoassay, and a live severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) microneutralisation assay (MNA80). Cellular responses were assessed using an ex-vivo IFN-γ enzyme-linked immunospot assay. The coprimary outcomes of the trial were efficacy, as measured by the number of cases of symptomatic, virologically confirmed COVID-19, and safety, as measured by the occurrence of serious adverse events. Analyses were by group allocation in participants who received the vaccine. Here, we report the preliminary findings on safety, reactogenicity, and cellular and humoral immune responses. This study is ongoing and is registered with ClinicalTrials.gov, NCT04400838, and ISRCTN, 15281137.
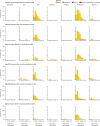
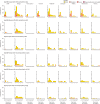
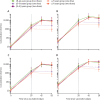
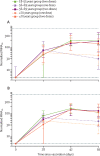
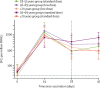
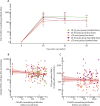
Findings: Between May 30 and Aug 8, 2020, 560 participants were enrolled: 160 aged 18-55 years (100 assigned to ChAdOx1 nCoV-19, 60 assigned to MenACWY), 160 aged 56-69 years (120 assigned to ChAdOx1 nCoV-19: 40 assigned to MenACWY), and 240 aged 70 years and older (200 assigned to ChAdOx1 nCoV-19: 40 assigned to MenACWY). Seven participants did not receive the boost dose of their assigned two-dose regimen, one participant received the incorrect vaccine, and three were excluded from immunogenicity analyses due to incorrectly labelled samples. 280 (50%) of 552 analysable participants were female. Local and systemic reactions were more common in participants given ChAdOx1 nCoV-19 than in those given the control vaccine, and similar in nature to those previously reported (injection-site pain, feeling feverish, muscle ache, headache), but were less common in older adults (aged ≥56 years) than younger adults. In those receiving two standard doses of ChAdOx1 nCoV-19, after the prime vaccination local reactions were reported in 43 (88%) of 49 participants in the 18-55 years group, 22 (73%) of 30 in the 56-69 years group, and 30 (61%) of 49 in the 70 years and older group, and systemic reactions in 42 (86%) participants in the 18-55 years group, 23 (77%) in the 56-69 years group, and 32 (65%) in the 70 years and older group. As of Oct 26, 2020, 13 serious adverse events occurred during the study period, none of which were considered to be related to either study vaccine. In participants who received two doses of vaccine, median anti-spike SARS-CoV-2 IgG responses 28 days after the boost dose were similar across the three age cohorts (standard-dose groups: 18-55 years, 20 713 arbitrary units [AU]/mL [IQR 13 898-33 550], n=39; 56-69 years, 16 170 AU/mL [10 233-40 353], n=26; and ≥70 years 17 561 AU/mL [9705-37 796], n=47; p=0·68). Neutralising antibody titres after a boost dose were similar across all age groups (median MNA80 at day 42 in the standard-dose groups: 18-55 years, 193 [IQR 113-238], n=39; 56-69 years, 144 [119-347], n=20; and ≥70 years, 161 [73-323], n=47; p=0·40). By 14 days after the boost dose, 208 (>99%) of 209 boosted participants had neutralising antibody responses. T-cell responses peaked at day 14 after a single standard dose of ChAdOx1 nCoV-19 (18-55 years: median 1187 spot-forming cells [SFCs] per million peripheral blood mononuclear cells [IQR 841-2428], n=24; 56-69 years: 797 SFCs [383-1817], n=29; and ≥70 years: 977 SFCs [458-1914], n=48).
Interpretation: ChAdOx1 nCoV-19 appears to be better tolerated in older adults than in younger adults and has similar immunogenicity across all age groups after a boost dose. Further assessment of the efficacy of this vaccine is warranted in all age groups and individuals with comorbidities.
Funding: UK Research and Innovation, National Institutes for Health Research (NIHR), Coalition for Epidemic Preparedness Innovations, NIHR Oxford Biomedical Research Centre, Thames Valley and South Midlands NIHR Clinical Research Network, and AstraZeneca.
Copyright © 2020 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY-NC-ND 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures
References
-
- WHO . World Health Organization; Geneva: Nov 13, 2020. Coronavirus disease (COVID-19) dashboard.https://covid19.who.int
-
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. - PubMed
-
- WHO . World Health Organization; Geneva: Nov 12, 2020. Draft landscape of COVID-19 vaccine candidates.https://www.who.int/publications/m/item/draft-landscape-of-covid-19-cand...
-
- WHO . World Health Organization; Geneva: April 29, 2020. WHO target product profiles for COVID-19 vaccines: version 3.https://www.who.int/docs/default-source/blue-print/who-target-product-pr...
-
- Joint Committee on Vaccination and Immunisation Priority groups for coronavirus (COVID-19) vaccination: advice from the JCVI, 25 September 2020. UK Government. Sept 25, 2020. https://www.gov.uk/government/publications/priority-groups-for-coronavir...
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

