Magnetic Resonance-Based Characterization of Myocardial Architecture
- PMID: 33220889
- PMCID: PMC8243505
- DOI: 10.1016/j.hfc.2020.08.007
Magnetic Resonance-Based Characterization of Myocardial Architecture
Abstract
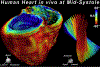
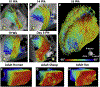
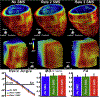
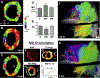
Advances in technology have made it possible to image the microstructure of the heart with diffusion-weighted magnetic resonance. The technique provides unique insights into the cellular architecture of the myocardium and how this is perturbed in a range of disease contexts. In this review, the physical basis of diffusion MRI and the challenges of implementing it in the beating heart are discussed. Cutting edge acquisition and analysis techniques, as well as the results of initial clinical studies, are reported.
Keywords: Cellular architecture; Diffusion tensor; Heart; Magnetic resonance; Microstructure; Myocardium; Tractography.
Copyright © 2020 The Author. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosure The Martinos Center for Biomedical Imaging receives research support from Siemens Medical, Erlangen Germany.
Figures


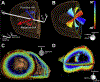

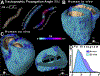

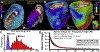



References
-
- Scollan DF, Holmes A, Winslow R, et al. Histological validation of myocardial microstructure obtained from diffusion tensor magnetic resonance imaging. Am J Physiol 1998;275(6 Pt 2):H2308–18. - PubMed
-
- Streeter DD Jr, Spotnitz HM, Patel DP, et al. Fiber orientation in the canine left ventricle during diastole and systole. Circ Res 1969;24(3):339–47. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

