Localized Optical Coherence Tomography Precursors of Macular Atrophy and Fibrotic Scar in the Comparison of Age-Related Macular Degeneration Treatments Trials
- PMID: 33221285
- PMCID: PMC7979472
- DOI: 10.1016/j.ajo.2020.11.002
Localized Optical Coherence Tomography Precursors of Macular Atrophy and Fibrotic Scar in the Comparison of Age-Related Macular Degeneration Treatments Trials
Abstract
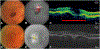
Purpose: To identify precursors of macular atrophy (MA) and of fibrotic scar (FS) in eyes treated with anti-vascular endothelial growth factor through pixel-mapping analysis of baseline optical coherence tomography (OCT).
Methods: Design: Cross-sectional analysis.
Setting: Multicenter clinical trial.
Patient population: 68 eyes from the Comparison of Age-Related Macular Degeneration Treatments Trials.
Intervention: Treatment with anti-vascular endothelial growth factor agents.
Main outcome measure: The percentage of MA or FS pixels with each OCT feature at baseline, and the odds ratio for baseline pixels with an OCT feature to develop MA or FS.
Results: Retinal pigment epithelium atrophy and photoreceptor loss on OCT were highly predictive of MA at that location at years 2 and 5 (P < .0001), but accounted for only 22.5% of the ensuing atrophy at year 2 and less at year 5. Among pixels of MA at year 2, 78% were preceded by thick drusen, 54% by subretinal macular neovascularization (MNV), and 22.5% by no detectable OCT features. MNV, subretinal hyperreflective material, pigment epithelial detachment, intraretinal fluid, and sub-retinal pigment epithelium fluid were predictive of FS at that location (P values <.05). More than 75% of the pixels of FS at years 2 and 5 were preceded by pixels of baseline MNV.
Conclusions: Most pixels of FS were preceded by components of neovascularization. Although one-quarter of MA was accounted for by pre-existing evidence of atrophy on OCT alone, the development of MA in areas of thick drusen, areas with and without subretinal MNV lesion, and areas without detectable OCT precursors argues that the development of MA is multifactorial and may follow, in part, a non-neovascular pathway.
Copyright © 2020 Elsevier Inc. All rights reserved.
Figures
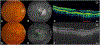


References
-
- Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group. Five-Year Outcomes with Anti-Vascular Endothelial Growth Factor Treatment of Neovascular Age-Related Macular Degeneration. Ophthalmology. 2016;123(8):1751–1761. doi: 10.1016/j.ophtha.2016.03.045 - DOI - PMC - PubMed
-
- Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K, SEVEN-UP Study Group. Seven-Year Outcomes in Ranibizumab-Treated Patients in ANCHOR, MARINA, and HORIZON. Ophthalmology. 2013;120(11):2292–2299. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

