Antibody response using six different serological assays in a completely PCR-tested community after a coronavirus disease 2019 outbreak-the CoNAN study
- PMID: 33221432
- PMCID: PMC7677041
- DOI: 10.1016/j.cmi.2020.11.009
Antibody response using six different serological assays in a completely PCR-tested community after a coronavirus disease 2019 outbreak-the CoNAN study
Abstract
Objectives: Due to a substantial proportion of asymptomatic and mild courses, many severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections remain unreported. Therefore, assessment of seroprevalence may detect the real burden of disease. We aimed to determine and characterize the rate of SARS-CoV-2 infections and the resulting seroprevalence in a defined population. The primary objective of the study was to assess SARS-CoV-2 antibody seroprevalence using six different IgG-detecting immunoassays. Secondary objectives of the study were: (a) to determine potential risk factors for symptomatic versus asymptomatic coronavirus disease 2019 courses, and (b) to investigate the rate of virus RNA-persistence.
Methods: CoNAN is a population-based cohort study performed in the community Neustadt am Rennsteig, Germany, which was quarantined from 22 March to 5 April after six SARS-CoV-2 cases were detected in the village's population. The SARS-CoV-2 outbreak comprised 51 cases and 3 deaths. The CoNAN study was performed from 13 May to 22 May 2020, 6 weeks after a SARS-CoV-2 outbreak.
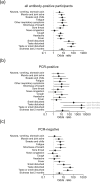
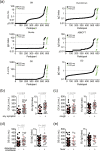
Results: We enrolled a total of 626 participants (71% of the community population) for PCR and antibody testing in the study. All actual SARS-CoV-2 PCR tests were negative. Fifty-two out of 620 (8.4%) participants had antibodies against SARS-CoV-2 in at least two different assays. There were 38 participants with previously PCR-confirmed SARS-CoV-2 infection. Of those, only 19 (50%) displayed anti-SARS-CoV-2 antibodies. We also show that antibody-positive participants with symptoms compatible with a respiratory tract infection had significantly higher antibody levels then asymptomatic participants (EU-assay: median 2.9 versus 7.2 IgG-index, p 0.002; DS-assay: median 45.2 versus 143 AU/mL, p 0.002). Persisting viral replication was not detected.
Conclusions: Our data question the relevance and reliability of IgG antibody testing to detect past SARS-CoV-2 infections 6 weeks after an outbreak. We conclude that assessing immunity for SARS-CoV-2 infection should not rely on antibody tests alone.
Keywords: Antibody response; Immunity; Quarantine; Severe acute respiratory syndrome coronavirus 2.
Copyright © 2020 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures
References
-
- WHO-2019 https://apps.who.int/iris/bitstream/handle/10665/331656/WHO-2019-nCoV-Se... Available from:
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

