Update on Clinical Ex Vivo Hematopoietic Stem Cell Gene Therapy for Inherited Monogenic Diseases
- PMID: 33221437
- PMCID: PMC7854296
- DOI: 10.1016/j.ymthe.2020.11.020
Update on Clinical Ex Vivo Hematopoietic Stem Cell Gene Therapy for Inherited Monogenic Diseases
Abstract
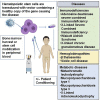
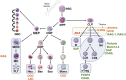
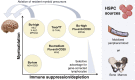
Gene transfer into autologous hematopoietic stem progenitor cells (HSPCs) has the potential to cure monogenic inherited disorders caused by an altered development and/or function of the blood system, such as immune deficiencies and red blood cell and platelet disorders. Gene-corrected HSPCs and their progeny can also be exploited as cell vehicles to deliver molecules into the circulation and tissues, including the central nervous system. In this review, we focus on the progress of clinical development of medicinal products based on HSPCs engineered and modified by integrating viral vectors for the treatment of monogenic blood disorders and metabolic diseases. Two products have reached the stage of market approval in the EU, and more are foreseen to be approved in the near future. Despite these achievements, several challenges remain for HSPC gene therapy (HSPC-GT) precluding a wider application of this type of gene therapy to a wider set of diseases while gene-editing approaches are entering the clinical arena.
Keywords: chemotherapy; gene editing; genetic disease; hematopoietic stem/progenitor cells; lentiviral vector; retroviral vector; transplantation.
Copyright © 2020 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The San Raffaele Telethon Institute for Gene Therapy (SR-Tiget) is a joint venture between Fondazione Telethon and Ospedale San Raffaele (OSR). Gene therapies for ADA-SCID, Wiskott-Aldrich syndrome (WAS), metachromatic leukodystrophy (MLD), β-thalassemia (BTHAL), and mucopolysaccharidosis I (MPS I) developed at SR-Tiget were licensed to Orchard Therapeutics (OTL) in 2018 and 2019. A.A. is the Principal Investigator of the above clinical trials.
Figures
References
-
- Slatter M.A., Gennery A.R. Hematopoietic cell transplantation in primary immunodeficiency–conventional and emerging indications. Expert Rev. Clin. Immunol. 2018;14:103–114. - PubMed
-
- Gaspar H.B., Cooray S., Gilmour K.C., Parsley K.L., Zhang F., Adams S., Bjorkegren E., Bayford J., Brown L., Davies E.G. Hematopoietic stem cell gene therapy for adenosine deaminase-deficient severe combined immunodeficiency leads to long-term immunological recovery and metabolic correction. Sci. Transl. Med. 2011;3:97ra80. - PubMed
-
- Aiuti A., Cattaneo F., Galimberti S., Benninghoff U., Cassani B., Callegaro L., Scaramuzza S., Andolfi G., Mirolo M., Brigida I. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N. Engl. J. Med. 2009;360:447–458. - PubMed
-
- Hacein-Bey-Abina S., Von Kalle C., Schmidt M., McCormack M.P., Wulffraat N., Leboulch P., Lim A., Osborne C.S., Pawliuk R., Morillon E. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

