Behavioural and neurochemical mechanisms underpinning the feeding-suppressive effect of GLP-1/CCK combinatorial therapy
- PMID: 33221554
- PMCID: PMC7720077
- DOI: 10.1016/j.molmet.2020.101118
Behavioural and neurochemical mechanisms underpinning the feeding-suppressive effect of GLP-1/CCK combinatorial therapy
Abstract
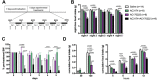
Objectives: Combinatorial therapies are under intense investigation to develop more efficient anti-obesity drugs; however, little is known about how they act in the brain to produce enhanced anorexia and weight loss. The goal of this study was to identify the brain sites and neuronal populations engaged during the co-administration of GLP-1R and CCK1R agonists, an efficient combination therapy in obese rodents.
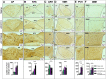
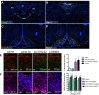
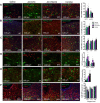
Methods: We measured acute and long-term feeding and body weight responses and neuronal activation patterns throughout the neuraxis and in specific neuronal subsets in response to GLP-1R and CCK1R agonists administered alone or in combination in lean and high-fat diet fed mice. We used PhosphoTRAP to obtain unbiased molecular markers for neuronal populations selectively activated by the combination of the two agonists.
Results: The initial anorectic response to GLP-1R and CCK1R co-agonism was mediated by a reduction in meal size, but over a few hours, a reduction in meal number accounted for the sustained feeding suppressive effects. The nucleus of the solitary tract (NTS) is one of the few brain sites where GLP-1R and CCK1R signalling interact to produce enhanced neuronal activation. None of the previously categorised NTS neuronal subpopulations relevant to feeding behaviour were implicated in this increased activation. However, we identified NTS/AP Calcrl+ neurons as treatment targets.
Conclusions: Collectively, these studies indicated that circuit-level integration of GLP-1R and CCK1R co-agonism in discrete brain nuclei including the NTS produces enhanced rapid and sustained appetite suppression and weight loss.
Keywords: CCK; Combinatorial therapy; GLP-1; Hunger; Nucleus of the solitary tract; Obesity; Satiety.
Copyright © 2020 The Authors. Published by Elsevier GmbH.. All rights reserved.
Figures

References
-
- Sadry S.A., Drucker D.J. Emerging combinatorial hormone therapies for the treatment of obesity and T2DM. Nature Reviews Endocrinology. 2013;9:425. - PubMed
-
- Ambery P., Parker V.E., Stumvoll M., Posch M.G., Heise T., Plum-Moerschel L. MEDI0382, a GLP-1 and glucagon receptor dual agonist, in obese or overweight patients with type 2 diabetes: a randomised, controlled, double-blind, ascending dose and phase 2a study. Lancet. 2018;391:2607–2618. - PubMed
-
- Tillner J., Posch M.G., Wagner F., Teichert L., Hijazi Y., Einig C. A novel dual glucagon-like peptide and glucagon receptor agonist SAR425899: Results of randomized, placebo-controlled first-in-human and first-in-patient trials. Diabetes, Obesity and Metabolism. 2019;21:120–128. - PubMed
-
- Frias J.P., Nauck M.A., Van J., Kutner M.E., Cui X., Benson C. 2018. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial; pp. 2180–2193. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

