T-tubule remodeling in human hypertrophic cardiomyopathy
- PMID: 33222034
- PMCID: PMC8332592
- DOI: 10.1007/s10974-020-09591-6
T-tubule remodeling in human hypertrophic cardiomyopathy
Abstract
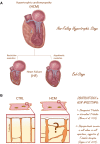
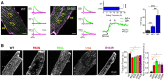
The highly organized transverse T-tubule membrane system represents the ultrastructural substrate for excitation-contraction coupling in ventricular myocytes. While the architecture and function of T-tubules have been well described in animal models, there is limited morpho-functional data on T-tubules in human myocardium. Hypertrophic cardiomyopathy (HCM) is a primary disease of the heart muscle, characterized by different clinical presentations at the various stages of its progression. Most HCM patients, indeed, show a compensated hypertrophic disease ("non-failing hypertrophic phase"), with preserved left ventricular function, and only a small subset of individuals evolves into heart failure ("end stage HCM"). In terms of T-tubule remodeling, the "end-stage" disease does not differ from other forms of heart failure. In this review we aim to recapitulate the main structural features of T-tubules during the "non-failing hypertrophic stage" of human HCM by revisiting data obtained from human myectomy samples. Moreover, by comparing pathological changes observed in myectomy samples with those introduced by acute (experimentally induced) detubulation, we discuss the role of T-tubular disruption as a part of the complex excitation-contraction coupling remodeling process that occurs during disease progression. Lastly, we highlight how T-tubule morpho-functional changes may be related to patient genotype and we discuss the possibility of a primitive remodeling of the T-tubule system in rare HCM forms associated with genes coding for proteins implicated in T-tubule structural integrity, formation and maintenance.
Keywords: Excitation–contraction coupling; Hypertrophic cardiomyopathy; T-tubules.
© 2020. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

