G6PD overexpression protects from oxidative stress and age-related hearing loss
- PMID: 33222382
- PMCID: PMC7744953
- DOI: 10.1111/acel.13275
G6PD overexpression protects from oxidative stress and age-related hearing loss
Abstract
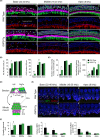
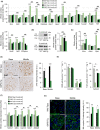
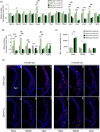
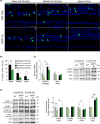
Aging of the auditory system is associated with the incremental production of reactive oxygen species (ROS) and the accumulation of oxidative damage in macromolecules, which contributes to cellular malfunction, compromises cell viability, and, ultimately, leads to functional decline. Cellular detoxification relies in part on the production of NADPH, which is an important cofactor for major cellular antioxidant systems. NADPH is produced principally by the housekeeping enzyme glucose-6-phosphate dehydrogenase (G6PD), which catalyzes the rate-limiting step in the pentose phosphate pathway. We show here that G6PD transgenic mice (G6PD-Tg), which show enhanced constitutive G6PD activity and NADPH production along life, have lower auditory thresholds than wild-type mice during aging, together with preserved inner hair cell (IHC) and outer hair cell (OHC), OHC innervation, and a conserved number of synapses per IHC. Gene expression of antioxidant enzymes was higher in 3-month-old G6PD-Tg mice than in wild-type counterparts, whereas the levels of pro-apoptotic proteins were lower. Consequently, nitration of proteins, mitochondrial damage, and TUNEL+ apoptotic cells were all lower in 9-month-old G6PD-Tg than in wild-type counterparts. Unexpectedly, G6PD overexpression triggered low-grade inflammation that was effectively resolved in young mice, as shown by the absence of cochlear cellular damage and macrophage infiltration. Our results lead us to propose that NADPH overproduction from an early stage is an efficient mechanism to maintain the balance between the production of ROS and cellular detoxification power along aging and thus prevents hearing loss progression.
Keywords: ARHL; NADPH; TrxR; aging; glutathione.
© 2020 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Benkafadar, N. , François, F. , Affortit, C. , Casas, F. , Ceccato, J.‐C. , Menardo, J. , Venail, F. , Malfroy‐Camine, B. , Puel, J.‐L. , & Wang, J. (2019). ROS‐induced activation of DNA damage responses drives senescence‐like state in postmitotic cochlear cells: Implication for hearing preservation. Molecular Neurobiology, 56(8), 5950–5969. 10.1007/s12035-019-1493-6 - DOI - PMC - PubMed
-
- Celaya, A. M. , Sánchez‐Pérez, I. , Bermúdez‐Muñoz, J. M. , Rodríguez‐de la Rosa, L. , Pintado‐Berninches, L. , Perona, R. , Murillo‐Cuesta, S. , & Varela‐Nieto, I. (2019). Deficit of mitogen‐activated protein kinase phosphatase 1 (DUSP1) accelerates progressive hearing loss. Elife, 8 10.7554/eLife.39159 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

