Improving the Stability of EGFR Inhibitor Cobalt(III) Prodrugs
- PMID: 33222438
- PMCID: PMC7724630
- DOI: 10.1021/acs.inorgchem.0c03083
Improving the Stability of EGFR Inhibitor Cobalt(III) Prodrugs
Abstract
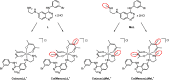
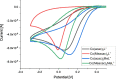
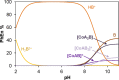
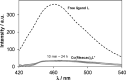
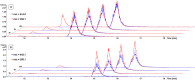
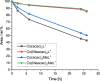
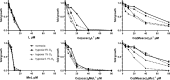
Although tyrosine kinase inhibitors (TKIs) have revolutionized cancer therapy in the past two decades, severe drawbacks such as strong adverse effects and drug resistance limit their clinical application. Prodrugs represent a valuable approach to overcoming these disadvantages by administration of an inactive drug with tumor-specific activation. We have recently shown that hypoxic prodrug activation is a promising strategy for a cobalt(III) complex bearing a TKI of the epidermal growth factor receptor (EGFR). The aim of this study was the optimization of the physicochemical properties and enhancement of the stability of this compound class. Therefore, we synthesized a series of novel derivatives to investigate the influence of the electron-donating properties of methyl substituents at the metal-chelating moiety of the EGFR inhibitor and/or the ancillary acetylacetonate (acac) ligand. To understand the effect of the different methylations on the redox properties, the newly synthesized complexes were analyzed by cyclic voltammetry and their behavior was studied in the presence of natural low-molecular weight reducing agents. Furthermore, it was proven that reduction to cobalt(II) resulted in a lower stability of the complexes and subsequent release of the coordinated TKI ligand. Moreover, the stability of the cobalt(III) prodrugs was investigated in blood serum as well as in cell culture by diverse cell and molecular biological methods. These analyses revealed that the complexes bearing the methylated acac ligand are characterized by distinctly enhanced stability. Finally, the cytotoxic activity of all new compounds was tested in cell culture under normoxic and various hypoxic conditions, and their prodrug nature could be correlated convincingly with the stability data. In summary, the performed chemical modifications resulted in new cobalt(III) prodrugs with strongly improved stabilities together with retained hypoxia-activatable properties.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

