microRNA-877 contributes to decreased non-small cell lung cancer cell growth via the PI3K/AKT pathway by targeting tartrate resistant acid phosphatase 5 activity
- PMID: 33222607
- PMCID: PMC7751652
- DOI: 10.1080/15384101.2020.1839697
microRNA-877 contributes to decreased non-small cell lung cancer cell growth via the PI3K/AKT pathway by targeting tartrate resistant acid phosphatase 5 activity
Abstract
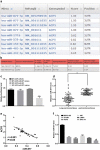
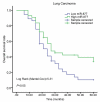
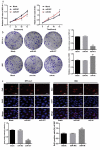
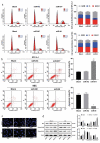
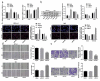
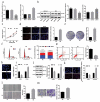
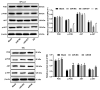
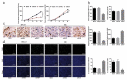
Non-small cell lung cancer (NSCLC) is a leading cause of cancer death in both men and women. microRNAs (miRs) can exert important functions in cancer development. However, the role of miR-877 in NSCLC as it relates to tartrate resistant acid phosphatase 5 (ACP5) is unknown. For this study, the gain-and-loss-of-function experiments were performed to explore the effects of miR-877 and ACP5 on NSCLC. miR-877 expression in LC and paracancerous tissues, lung epithelial cell line and NSCLC cell lines was detected, and the association between miR-877 expression and clinical features of LC patients was analyzed. The levels of ACP5, epithelial-mesenchymal transition (EMT) markers and apoptosis-related proteins were measured. In vivo experiments were conducted for further validation. Consequently, we found that miR-877 expression was lowered in LC tissues and cell lines, and correlated with clinical stage, differentiation, lymph node metastasis and prognosis of NSCLC patients. Additionally, miR-877 was determined to inhibit ACP5 activity, and miR-877 downregulated the PI3K/AKT pathway by silencing ACP5. Furthermore, overexpression of miR-877 inhibited the viability, migration, invasion and EMT of NSCLC cells, but promoted cell apoptosis. In conclusion, miR-877 overexpression inhibited malignant biological behaviors of NSCLC cells by downregulating ACP5 and inactivating the PI3K/AKT pathway.
Keywords: Non-small cell lung cancer; PI3K/AKT pathway; microRNA-877; tartrate resistant acid phosphatase 5.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Mulherkar R, Grewal AS, Berman AT.. Emerging role of immunotherapy in locally advanced non-small cell lung cancer. Clin Adv Hematol Oncol. 2020;18(4):212–217. - PubMed
-
- Reck M, Popat S, Reinmuth N, et al. Metastatic non-small-cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):iii27–39. . - PubMed
-
- Fajersztajn L, Veras M, Barrozo LV, et al. Air pollution: a potentially modifiable risk factor for lung cancer. Nat Rev Cancer. 2013;13(9):674–678. . - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
