Regional binding of tau and amyloid PET tracers in Down syndrome autopsy brain tissue
- PMID: 33222700
- PMCID: PMC7682014
- DOI: 10.1186/s13024-020-00414-3
Regional binding of tau and amyloid PET tracers in Down syndrome autopsy brain tissue
Abstract
Introduction: Tau pathology is a major age-related event in Down syndrome with Alzheimer's disease (DS-AD). Although recently, several different Tau PET tracers have been developed as biomarkers for AD, these tracers showed different binding properties in Alzheimer disease and other non-AD tauopathies. They have not been yet investigated in tissue obtained postmortem for DS-AD cases. Here, we evaluated the binding characteristics of two Tau PET tracers (3H-MK6240 and 3H-THK5117) and one amyloid (3H-PIB) ligand in the medial frontal gyrus (MFG) and hippocampus (HIPP) in tissue from adults with DS-AD and DS cases with mild cognitive impairment (MCI) compared to sporadic AD.
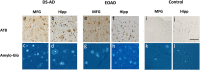
Methods: Tau and amyloid autoradiography were performed on paraffin-embedded sections. To confirm respective ligand targets, adjacent sections were immunoreacted for phospho-Tau (AT8) and stained for amyloid staining using Amylo-Glo.
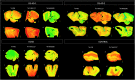
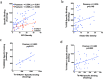
Results: The two Tau tracers showed a significant correlation with each other and with AT8, suggesting that both tracers were binding to Tau deposits. 3H-MK6240 Tau binding correlated with AT8 immunostaining but to a lesser degree than the 3H-THK5117 tracer, suggesting differences in binding sites between the two Tau tracers. 3H-THK5117, 3H-MK6240 and 3H-PIB displayed dense laminar binding in the HIPP and MFG in adult DS brains. A regional difference in Tau binding between adult DS and AD was observed suggesting differential regional Tau deposition in adult DS compared to AD, with higher THK binding density in the MFG in adult with DS compared to AD. No significant correlation was found between 3H-PIB and Amylo-Glo staining in adult DS brains suggesting that the amyloid PIB tracer binds to additional sites.
Conclusions: This study provides new insights into the regional binding distribution of a first-generation and a second-generation Tau tracer in limbic and neocortical regions in adults with DS, as well as regional differences in Tau binding in adult with DS vs. those with AD. These findings provide new information about the binding properties of two Tau radiotracers for the detection of Tau pathology in adults with DS in vivo and provide valuable data regarding Tau vs. amyloid binding in adult DS compared to AD.
Keywords: Alzheimer’s disease; Down syndrome; Neurofibrillary tangles; Neuropathology; PET tracers.
Conflict of interest statement
The authors do not report any conflicts of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

