Primary polydipsia: Update
- PMID: 33222764
- PMCID: PMC7683824
- DOI: 10.1016/j.beem.2020.101469
Primary polydipsia: Update
Abstract
In primary polydipsia pathologically high levels of water intake physiologically lower arginine vasopressin (AVP) secretion, and in this way mirror the secondary polydipsia in diabetes insipidus in which pathologically low levels of AVP (or renal responsiveness to AVP) physiologically increase water intake. Primary polydipsia covers several disorders whose clinical features and significance, risk factors, pathophysiology and treatment are reviewed here. While groupings may appear somewhat arbitrary, they are associated with distinct alterations in physiologic parameters of water balance. The polydipsia is typically unrelated to homeostatic regulation of water intake, but instead reflects non-homeostatic influences. Recent technological advances, summarized here, have disentangled functional neurocircuits underlying both homeostatic and non-homeostatic physiologic influences, which provides an opportunity to better define the mechanisms of the disorders. We summarize this recent literature, highlighting hypothalamic circuitry that appears most clearly positioned to contribute to primary polydipsia. The life-threatening water imbalance in psychotic disorders is caused by an anterior hippocampal induced stress-diathesis that can be reproduced in animal models, and involves phylogenetically preserved pathways that appear likely to include one or more of these circuits. Ongoing translational neuroscience studies in these animal models may potentially localize reversible pathological changes which contribute to both the water imbalance and psychotic disorder.
Keywords: arginine vasopressin; compulsive water drinking; hyponatremia; psychogenic polydipsia; psychosis-intermittent hyponatremia-polydipsia syndrome; schizophrenia.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures

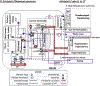
 ) lamina terminalis nuclei (SFO, OVLT) outside the blood brain barrier and via relays in the brainstem which project to the third LT nucleus (MnPO). The MnPO integrates signals from the SFO and OVLT and is the primary structure which coordinates fluid intake, such that any output from glutamatergic MnPO neurons may be sufficient to over-ride satiety signals (solid purple efferents). B. Non-homeostatic influences that anticipate water intake and protect from overhydration act largely via gabaergic projections (
) lamina terminalis nuclei (SFO, OVLT) outside the blood brain barrier and via relays in the brainstem which project to the third LT nucleus (MnPO). The MnPO integrates signals from the SFO and OVLT and is the primary structure which coordinates fluid intake, such that any output from glutamatergic MnPO neurons may be sufficient to over-ride satiety signals (solid purple efferents). B. Non-homeostatic influences that anticipate water intake and protect from overhydration act largely via gabaergic projections ( ) in the LT nuclei. Of particular interest is the oropharyngeal projection which seems to regulate the dopamine release that occurs with swallowing and somehow can operate independently of satiation, likely through poorly understood projections to the lateral hypothalamic area (dashed purple line with ?). C. Non-homeostatic projections also promote intake in advance of need for water. These include eating where there are multiple overlapping pathways between the two appetitive systems. D. Efferents from the MnPO relay via the LHA and PVT to the cortical and subcortical structures that regulate drinking behavior. Both pathways appear capable of enhancing intake even in the presence of satiety signals. Potential pathways and mechanisms relevant to the effects of psychologic stress on intake are discussed in the text. Abbreviations: AH: Anterior hypothalamus; LHA: Lateral hypothalamic area; MnPO: median preoptic nucleus; NTS: nucleus of the solitary tract; OVLT: organum vasculosum of the lateral terminalis; PBN: parabrachial nucleus; PP: posterior pituitary; PVN: paraventricular nucleus; PVT: paraventricular thalamus; SCN:suprachiasmatic nucleus SFO: sub-fornical organ; SON:supraoptic nucleus.
) in the LT nuclei. Of particular interest is the oropharyngeal projection which seems to regulate the dopamine release that occurs with swallowing and somehow can operate independently of satiation, likely through poorly understood projections to the lateral hypothalamic area (dashed purple line with ?). C. Non-homeostatic projections also promote intake in advance of need for water. These include eating where there are multiple overlapping pathways between the two appetitive systems. D. Efferents from the MnPO relay via the LHA and PVT to the cortical and subcortical structures that regulate drinking behavior. Both pathways appear capable of enhancing intake even in the presence of satiety signals. Potential pathways and mechanisms relevant to the effects of psychologic stress on intake are discussed in the text. Abbreviations: AH: Anterior hypothalamus; LHA: Lateral hypothalamic area; MnPO: median preoptic nucleus; NTS: nucleus of the solitary tract; OVLT: organum vasculosum of the lateral terminalis; PBN: parabrachial nucleus; PP: posterior pituitary; PVN: paraventricular nucleus; PVT: paraventricular thalamus; SCN:suprachiasmatic nucleus SFO: sub-fornical organ; SON:supraoptic nucleus.References
-
- Mercier-Guidez E & Loas G. Polydipsia and water intoxication in 353 psychiatric inpatients:an epidemiological and psychopathological study. European Journal of Psychiatry 2000; 15: 306–311. - PubMed
-
- Vieweg WVR. Treatment strategies in the polydipsia-hyponatremia syndrome. The Journal of Clinical Psychiatry 1994; 55(4): 154–160. - PubMed
-
- Benton D Executive summary and conclusions from the European Hydration Institute Expert Conference on human hydration, health, and performance. Nutrition Reviews 2015; 73 (Suppl. 2): 148–150. - PubMed
-
- Barlow ED & De Wardener HE. Compulsive water drinking. Quarterly Journal of Medicine 1959; 28: 235–258. - PubMed
-
- McKenna K & Thompson C. Osmoregulation in clinical disorders of thirst appreciation. Clinical Endocrinology 1998; 49:139–152. - PubMed
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

