Acceptability, safety, and patterns of use of oral tenofovir disoproxil fumarate and emtricitabine for HIV pre-exposure prophylaxis in South African adolescents: an open-label single-arm phase 2 trial
- PMID: 33222803
- PMCID: PMC9832157
- DOI: 10.1016/S2352-4642(20)30248-0
Acceptability, safety, and patterns of use of oral tenofovir disoproxil fumarate and emtricitabine for HIV pre-exposure prophylaxis in South African adolescents: an open-label single-arm phase 2 trial
Abstract
Background: HIV incidence among adolescents in southern Africa remains unacceptably high. Pre-exposure prophylaxis (PrEP) is an effective HIV prevention intervention but there are few data on its implementation among adolescents. We aimed to investigate the safety, feasibility, and acceptability of PrEP with oral tenofovir disoproxil fumarate and emtricitabine as part of a comprehensive HIV prevention package in an adolescent population in South Africa.
Methods: This open-label single-arm phase 2 study (PlusPills) was done in two research clinics in Cape Town and Johannesburg, South Africa. Adolescents aged 15-19 years were recruited into the study through recruitment events and outreach in the community. Potential participants were eligible for enrolment if they reported being sexually active. Exclusion criteria were a positive test for HIV or pregnancy at enrolment, breastfeeding, or any relevant co-morbidities. Participants were given oral tenofovir disoproxil fumarate and emtricitabine for PrEP to take daily for the first 12 weeks and were then given the choice to opt in or out of PrEP use at three monthly intervals during scheduled clinic visits. Participants were invited to monthly visits for adherence counselling and HIV testing during the study period. The primary outcomes were acceptability, use, and safety of PrEP. Acceptability was measured by the proportion of participants who reported willingness to take up PrEP and remain on PrEP at each study timepoint. Use was defined as the number of participants who continued to use PrEP after the initial 12-week period until the end of the study (week 48). Safety was measured by grade 2, 3, and 4 laboratory and clinical adverse events using the Division of AIDS table for grading the severity of adult and paediatric adverse events, version 1.0. Dried blood spot samples were collected at each study time-point to measure tenofovir diphosphate concentrations. This trial is registered with ClinicalTrials.gov, NCT02213328.
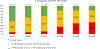
Findings: Between April 28, 2015, and Nov 11, 2016, 244 participants were screened, and 148 participants were enrolled (median age was 18 years; 99 participants [67%] were female) and initiated PrEP. PrEP was stopped by 26 of the 148 (18%) participants at 12 weeks. Cumulative PrEP opt-out, from the total cohort, was 41% (60 of 148 participants) at week 24 and 43% (63 of 148 participants) at week 36. PrEP was well tolerated with only minor adverse events (grade 2) thought to be related to study drug, which included headache (n=4, 3%), gastrointestinal upset (n=8, 5%), and skin rash (n=2, 1%). Two participants (1%) experienced grade 3 weight loss, which was deemed related to the study drug and resolved fully when PrEP was discontinued. Tenofovir diphosphate concentrations were detectable (>16 fmol/punch) in dried blood spot samples in 108 (92%) of 118 participants who reported PrEP use at week 12, in 74 (74%) of 100 participants at week 24, and in 22 (59%) of 37 participants by the study end at week 48.
Interpretation: In this cohort of self-selected South African adolescents at risk of HIV acquisition, PrEP appears safe and tolerable in those who continued use. PrEP use decreased throughout the course of the study as the number of planned study visits declined. Adolescents in southern Africa needs access to PrEP with tailored adherence support and possibly the option for more frequent and flexible visit schedules.
Funding: National Institute of Allergy and Infectious Diseases of the US National Institutes of Health.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures
Comment in
-
Oral PrEP in adolescents in sub-Saharan Africa.Lancet Child Adolesc Health. 2020 Dec;4(12):854-855. doi: 10.1016/S2352-4642(20)30340-0. Epub 2020 Oct 24. Lancet Child Adolesc Health. 2020. PMID: 33222801 No abstract available.
References
-
- UNAIDS. Joint United Nations Programme on HIV/AIDS (UNAIDS). [Internet]. UNAIDS; 2018. Switzerland [updated 2019 Jul 20; cited 2020 26 Feb]. Available from: http://www.unaids.org/sites/default/files/media_asset/20170720_Data_book...
-
- UNICEF. ALL IN to end the adolescent AIDS epidemic. Switzerland. UNICEF; [updated 2016 Dec 16; cited 2019 Apr 9]. Available from: http://www.unaids.org/sites/default/files/media_asset/ALLIN2016ProgressR...
-
- Harrison A, Colvin CJ, Kuo C, Swartz Alison Swartz, Lurie M. Sustained High HIV Incidence in Young Women in Southern Africa: Social, Behavioral, and Structural Factors and Emerging Intervention Approaches. Curr HIV/AIDS Rep. [Internet]. 2015. Dec [cited 2019 Apr 09]; 12(2):207–215. Available from: URL 10.1007/s11904-015-0261-0 - DOI - PMC - PubMed
-
- UNAIDS. Joint United Nations Programme on HIV/AIDS. AIDS by the numbers, AIDS is not over, but it can be. Switzerland. UNAIDS; 2016. [updated 2016 Nov 21; cited 2019 Apr 9]. Available from: http://www.unaids.org/en/resources/documents/2016/AIDS-by-the-numbers
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous