Photoreceptor Degeneration in Pro23His Transgenic Rats (Line 3) Involves Autophagic and Necroptotic Mechanisms
- PMID: 33224023
- PMCID: PMC7670078
- DOI: 10.3389/fnins.2020.581579
Photoreceptor Degeneration in Pro23His Transgenic Rats (Line 3) Involves Autophagic and Necroptotic Mechanisms
Abstract
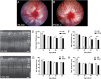
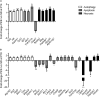
Photoreceptor death contributes to 50% of irreversible vision loss in the western world. Pro23His (P23H) transgenic albino rat strains are widely used models for the most common rhodopsin gene mutation associated with the autosomal dominant form of retinitis pigmentosa. However, the mechanism(s) by which photoreceptor death occurs are not well understood and were the principal aim of this study. We first used electroretinogram recording and optical coherence tomography to confirm the time course of functional and structural loss. Electroretinogram analyses revealed significantly decreased rod photoreceptor (a-wave), bipolar cell (b-wave) and amacrine cell responses (oscillatory potentials) from P30 onward. The cone-mediated b-wave was also decreased from P30. TUNEL analysis showed extensive cell death at P18, with continued labeling detected until P30. Focused gene expression arrays indicated activation of, apoptosis, autophagy and necroptosis in whole retina from P14-18. However, analysis of mitochondrial permeability changes (ΔΨm) using JC-1 dye, combined with immunofluorescence markers for caspase-dependent (cleaved caspase-3) and caspase-independent (AIF) cell death pathways, indicated mitochondrial-mediated cell death was not a major contributor to photoreceptor death. By contrast, reverse-phase protein array data combined with RIPK3 and phospho-MLKL immunofluorescence indicated widespread necroptosis as the predominant mechanism of photoreceptor death. These findings highlight the complexity of mechanisms involved in photoreceptor death in the Pro23His rat model of degeneration and suggest therapies that target necroptosis should be considered for their potential to reduce photoreceptor death.
Keywords: apoptosis; autophagy; cell death; necroptosis; retinitis pigmentosa photoreceptor.
Copyright © 2020 Kakavand, Jobling, Greferath, Vessey, de Iongh and Fletcher.
Figures







Similar articles
-
Cell Death Pathways in Mutant Rhodopsin Rat Models Identifies Genotype-Specific Targets Controlling Retinal Degeneration.Mol Neurobiol. 2019 Mar;56(3):1637-1652. doi: 10.1007/s12035-018-1192-8. Epub 2018 Jun 18. Mol Neurobiol. 2019. PMID: 29911255
-
Regressive and reactive changes in the connectivity patterns of rod and cone pathways of P23H transgenic rat retina.Neuroscience. 2004;127(2):301-17. doi: 10.1016/j.neuroscience.2004.04.042. Neuroscience. 2004. PMID: 15262321
-
A diffusible factor from normal retinal cells promotes rod photoreceptor survival in an in vitro model of retinitis pigmentosa.J Neurobiol. 1999 Jun 15;39(4):475-90. J Neurobiol. 1999. PMID: 10380070
-
P23H rhodopsin transgenic rat: correlation of retinal function with histopathology.Invest Ophthalmol Vis Sci. 2000 Sep;41(10):3200-9. Invest Ophthalmol Vis Sci. 2000. PMID: 10967084
-
Changes in the photoreceptor mosaic of P23H-1 rats during retinal degeneration: implications for rod-cone dependent survival.Invest Ophthalmol Vis Sci. 2013 Aug 28;54(8):5888-900. doi: 10.1167/iovs.13-12643. Invest Ophthalmol Vis Sci. 2013. PMID: 23908186
Cited by
-
Mechanisms of Rhodopsin-Related Inherited Retinal Degeneration and Pharmacological Treatment Strategies.Cells. 2025 Jan 4;14(1):49. doi: 10.3390/cells14010049. Cells. 2025. PMID: 39791750 Free PMC article. Review.
-
Astragaloside A Protects Against Photoreceptor Degeneration in Part Through Suppressing Oxidative Stress and DNA Damage-Induced Necroptosis and Inflammation in the Retina.J Inflamm Res. 2022 May 20;15:2995-3020. doi: 10.2147/JIR.S362401. eCollection 2022. J Inflamm Res. 2022. PMID: 35645574 Free PMC article.
-
Inducible Pluripotent Stem Cells to Model and Treat Inherited Degenerative Diseases of the Outer Retina: 3D-Organoids Limitations and Bioengineering Solutions.Cells. 2021 Sep 20;10(9):2489. doi: 10.3390/cells10092489. Cells. 2021. PMID: 34572137 Free PMC article. Review.
-
Inherited Retinal Dystrophies: Role of Oxidative Stress and Inflammation in Their Physiopathology and Therapeutic Implications.Antioxidants (Basel). 2022 May 30;11(6):1086. doi: 10.3390/antiox11061086. Antioxidants (Basel). 2022. PMID: 35739983 Free PMC article. Review.
-
Autophagy disruption and mitochondrial stress precede photoreceptor necroptosis in multiple mouse models of inherited retinal disorders.Nat Commun. 2025 Apr 29;16(1):4024. doi: 10.1038/s41467-025-59165-8. Nat Commun. 2025. PMID: 40301324 Free PMC article.
References
-
- Barber A. J., Nakamura M., Wolpert E. B., Reiter C. E., Seigel G. M., Antonetti D. A., et al. (2001). Insulin rescues retinal neurons from apoptosis by a phosphatidylinositol 3-kinase/Akt-mediated mechanism that reduces the activation of caspase-3. J. Biol. Chem. 276 32814–32821. 10.1074/jbc.M104738200 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

