Symptomatic COVID-19 in advanced-cancer patients treated with immune-checkpoint inhibitors: prospective analysis from a multicentre observational trial by FICOG
- PMID: 33224275
- PMCID: PMC7649863
- DOI: 10.1177/1758835920968463
Symptomatic COVID-19 in advanced-cancer patients treated with immune-checkpoint inhibitors: prospective analysis from a multicentre observational trial by FICOG
Abstract
Background: This prospective, multicentre, observational INVIDIa-2 study is investigating the clinical efficacy of influenza vaccination in advanced-cancer patients receiving immune-checkpoint inhibitors (ICIs), enrolled in 82 Italian centres, from October 2019 to January 2020. The primary endpoint was the incidence of influenza-like illness (ILI) until 30 April 2020. All the ILI episodes, laboratory tests, complications, hospitalizations and pneumonitis were recorded. Therefore, the study prospectively recorded all the COVID-19 ILI events.
Patients and methods: Patients were included in this non-prespecified COVID-19 analysis, if alive on 31 January 2020, when the Italian government declared the national emergency. The prevalence of confirmed COVID-19 cases was detected as ILI episode with laboratory confirmation of SARS-CoV-2. Cases with clinical-radiological diagnosis of COVID-19 (COVID-like ILIs), were also reported.
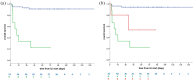
Results: Out of 1257 enrolled patients, 955 matched the inclusion criteria for this unplanned analysis. From 31 January to 30 April 2020, 66 patients had ILI: 9 of 955 cases were confirmed COVID-19 ILIs, with prevalence of 0.9% [95% confidence interval (CI): 0.3-2.4], a hospitalization rate of 100% and a mortality rate of 77.8%. Including 5 COVID-like ILIs, the overall COVID-19 prevalence was 1.5% (95% CI: 0.5-3.1), with 100% hospitalization and 64% mortality. The presence of elderly, males and comorbidities was significantly higher among patients vaccinated against influenza versus unvaccinated (p = 0.009, p < 0.0001, p < 0.0001). Overall COVID-19 prevalence was 1.2% for vaccinated (six of 482 cases, all confirmed) and 1.7% for unvaccinated (8 of 473, 3 confirmed COVID-19 and 5 COVID-like), p = 0.52. The difference remained non-significant, considering confirmed COVID-19 only (p = 0.33).
Conclusion: COVID-19 has a meaningful clinical impact on the cancer-patient population receiving ICIs, with high prevalence, hospitalization and an alarming mortality rate among symptomatic cases. Influenza vaccination does not protect from SARS-CoV-2 infection.
Keywords: COVID-19; SARS-CoV-2; cancer patients; immune-checkpoint inhibitors; influenza-like illness.
© The Author(s), 2020.
Conflict of interest statement
Conflict of interest statement: The Federation of Italian Cooperative Oncology Groups (FICOG) received funding for the present study by Seqirus and Roche S.p.A.; the Federation also received funding during the conduct of the present study by Astra Zeneca, Bristol Myers Squibb (BMS), Sanofi. Melissa Bersanelli received funding for the present study by Seqirus and Roche S.p.A. (FICOG as Institution, no personal fees). She also received, outside the present work, research funding from Pfizer and Novartis (Institution), honoraria as speaker at scientific events (personal fees) by Astra Zeneca, Bristol Myers Squibb (BMS), Novartis and Pfizer; as consultant for advisory role (personal fees) by Novartis, BMS and Pfizer. Ugo De Giorgi received honoraria from AstraZeneca, Roche, MSD, Pfizer, GSK, Clovis, Incyte and research funding from Roche, AstraZeneca, MSD, Pfizer. Dr Di Maio reports personal fees from Bristol Myers Squibb, personal fees from Merck Sharp & Dohme, personal fees from AstraZeneca, personal fees from Janssen, personal fees from Astellas, personal fees from Pfizer, personal fees from Eisai, personal fees from Takeda, grants from Tesaro GSK, outside the submitted work. Sebastiano Buti received honoraria as speaker at scientific events and advisory role by BMS, Pfizer; MSD, Ipsen, Roche S.p.A., Eli Lilly, AstraZeneca and Novartis; he also received research funding from Novartis. Marcello Tiseo received honoraria (personal fees) by MSD, BMS, Boehringer (BI), Takeda, AstraZeneca, and research funding by AstraZeneca (Institution). Vieri Scotti participated, with personal fees, to advisory boards and speaker’s bureaus for Roche S.p.A. Dr Cortellini reports grants from AstraZeneca, grants from MSD, grants from BMS, grants from Roche, during the conduct of the study; grants from AstraZeneca, grants from MSD, grants from BMS, grants from Roche, grants from Novartis, outside the submitted work. Saverio Cinieri declared international board for Eli Lilly international. Paolo Andrea Zucali acts in a consultant or advisory role for Sanofi, BMS, Pfizer, MSD, Astellas, Janssen, Ipsen, Novartis, all outside the scope of work. Sergio Bracarda declares to have acted as advisory board member (uncompensated) for: Janssen, Astellas, Pfizer, MSD, BMS, Merck, AstraZeneca, AAA, Ipsen, Bayer, Roche/Genentech. Francesco Carozza declared personal fees from Janssen. Sara Pilotto reports personal fees from AstraZeneca, Eli Lilly, Boehringer Ingelheim, Merk & Co, Roche, outside the submitted work. All remaining authors have declared no conflicts of interest.
Figures


References
-
- Johns Hopkins Coronavirus Resource Center. Coronavirus COVID-19 global cases by the Center for Systems Science and Engineering. https://coronavirus.jhu.edu/map.html (2020, accessed 15 July 2020).
LinkOut - more resources
Full Text Sources
Miscellaneous

