Heterologous prime-boost immunization co-targeting dual antigens inhibit tumor growth and relapse
- PMID: 33224629
- PMCID: PMC7657584
- DOI: 10.1080/2162402X.2020.1841392
Heterologous prime-boost immunization co-targeting dual antigens inhibit tumor growth and relapse
Abstract
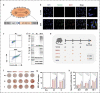
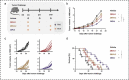
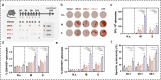
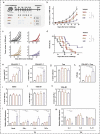
Therapeutic cancer vaccines aim to induce an effective immune response against cancer, and the effectiveness of these vaccines is influenced by the choice of immunogen, vaccine type, and immunization strategy. Although treatment with cancer vaccines can improve tumor burden and survival, in most animal studies, it is challenging to achieve a complete response against tumor growth and recurrence, without the use of other therapies in combination. Here, we present a novel approach where dual antigens (survivin and MUC1) are co-targeted using three DNA vaccines, followed by a single booster of a recombinant modified vaccinia Ankara (MVA) vaccine. This heterologous vaccination strategy induced higher levels of interferon (IFN)-γ-secretion and stronger antigen-specific T-cell responses than those induced individually by the DNA vaccines and the MVA vaccine in mice. This strategy also increased the number of active tumor-infiltrating T cells that efficiently inhibit tumor growth in tumor-bearing mice. Heterologous DNA prime-MVA boost immunization was capable of inducing a robust antigen-specific immune-memory, as seen from the resistance to subsequent survivin- and MUC1-expressing tumors. Moreover, the therapeutic effects of DNA prime-MVA boost and DNA prime-adenovirus boost strategies were compared. DNA prime-MVA boost immunization performed better, as indicated by the T effector ratio and the induction of Th1 immunity. This study provides the basis for the use of heterologous DNA prime-MVA boost vaccination regime targeting two antigens simultaneously as a promising immunotherapeutic strategy against cancer.
Keywords: Immunotherapy; MUC1; cancer vaccine; prime-boost; survivin.
© 2020 The Author(s). Published with license by Taylor & Francis Group, LLC.
Conflict of interest statement
The authors report no conflict of interest.
Figures


Similar articles
-
Potent Anti-hepatitis C Virus (HCV) T Cell Immune Responses Induced in Mice Vaccinated with DNA-Launched RNA Replicons and Modified Vaccinia Virus Ankara-HCV.J Virol. 2019 Mar 21;93(7):e00055-19. doi: 10.1128/JVI.00055-19. Print 2019 Apr 1. J Virol. 2019. PMID: 30674625 Free PMC article.
-
A multigene HIV type 1 subtype C modified vaccinia Ankara (MVA) vaccine efficiently boosts immune responses to a DNA vaccine in mice.AIDS Res Hum Retroviruses. 2008 Feb;24(2):207-17. doi: 10.1089/aid.2007.0206. AIDS Res Hum Retroviruses. 2008. PMID: 18240963
-
Performance of Homologous and Heterologous Prime-Boost Immunization Regimens of Recombinant Adenovirus and Modified Vaccinia Virus Ankara Expressing an Ag85B-TB10.4 Fusion Protein against Mycobacterium tuberculosis.J Microbiol Biotechnol. 2018 Jun 28;28(6):1022-1029. doi: 10.4014/jmb.1712.12064. J Microbiol Biotechnol. 2018. PMID: 29847865
-
DNA-based vaccines for malaria: a heterologous prime-boost immunisation strategy.Dev Biol (Basel). 2000;104:171-9. Dev Biol (Basel). 2000. PMID: 11713817 Review.
-
Synergistic DNA-MVA prime-boost vaccination regimes for malaria and tuberculosis.Vaccine. 2006 May 22;24(21):4554-61. doi: 10.1016/j.vaccine.2005.08.048. Epub 2005 Aug 24. Vaccine. 2006. PMID: 16150517 Review.
Cited by
-
Fast DNA Vaccination Strategy Elicits a Stronger Immune Response Dependent on CD8+CD11c+ Cell Accumulation.Front Oncol. 2021 Dec 7;11:752444. doi: 10.3389/fonc.2021.752444. eCollection 2021. Front Oncol. 2021. PMID: 34950581 Free PMC article.
-
The XCL1-Mediated DNA Vaccine Targeting Type 1 Conventional Dendritic Cells Combined with Gemcitabine and Anti-PD1 Antibody Induces Potent Antitumor Immunity in a Mouse Lung Cancer Model.Int J Mol Sci. 2024 Feb 4;25(3):1880. doi: 10.3390/ijms25031880. Int J Mol Sci. 2024. PMID: 38339158 Free PMC article.
-
New insights for the development of efficient DNA vaccines.Microb Biotechnol. 2024 Nov;17(11):e70053. doi: 10.1111/1751-7915.70053. Microb Biotechnol. 2024. PMID: 39545748 Free PMC article. Review.
-
B16 Membrane-Coated Vesicles for Combined Photodynamic Therapy and Immunotherapy Shift Immune Microenvironment of Melanoma.Int J Nanomedicine. 2022 Feb 22;17:855-868. doi: 10.2147/IJN.S338488. eCollection 2022. Int J Nanomedicine. 2022. PMID: 35360006 Free PMC article.
-
Hyperosmotic cold shock mouse melanoma cells encapsulated with doxorubicin for targeted treatment of melanoma.Front Oncol. 2024 May 1;14:1403719. doi: 10.3389/fonc.2024.1403719. eCollection 2024. Front Oncol. 2024. PMID: 38751816 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
